Chemical Change: Describe, analyse and apply quantitative aspects of change
Unit 1: Chemical equations
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Write chemical equations.
- Identify the reactants and products.
- Understand that mass is conserved during a chemical reaction.
- Balance chemical equations.
What you should know
Before you start this unit, make sure you can:
- Understand how to write chemical formulae. Refer to level 2 subject outcome 5.4 unit 3 to revise this.
- Understand and can represent the electron configuration of an element. Refer to level 2 subject outcome 5.3 unit 1 to revise this.
Introduction
Parts of the text in this unit were sourced from Siyavula Physical Science Gr 10 Learner’s Book, Chapter14, released under a CC-BY licence.
Chemical equations show what is being used to make new substances. The purpose of writing a balanced chemical equation is to know the reactants (starting materials) and products (end results) that occur and the ratios in which they react so you can calculate how much of the reactants you need and how much of the products will be formed.
Writing chemical formulae
Before you learn to write balanced chemical equations, it is very important to know the chemical symbols for common elements in the periodic table, so that you can write chemical equations and recognise different compounds. Using chemical symbols, you can then write for compounds.
A chemical formula is a concise way of giving information about the atoms that make up a particular chemical compound. A chemical formula shows each element by its symbol and also shows the ratio of the atoms of each element in that compound. The number of atoms (if greater than one) is shown as a subscript. For example, water is made up of [latex]\scriptsize 2[/latex] atoms of hydrogen and [latex]\scriptsize 1[/latex] atom of oxygen and has the chemical formula: [latex]\scriptsize {{\text{H}}_{2}}\text{O}[/latex]. The subscript [latex]\scriptsize 2[/latex] shows that for every atom of oxygen, [latex]\scriptsize 2[/latex] atoms of hydrogen are needed. This is because oxygen has an electron configuration of [latex]\scriptsize 2,6[/latex] so it needs to share/gain [latex]\scriptsize 2[/latex] electrons for a stable electron configuration.
Prefixes and suffixes in chemical formulae
The chemical suffix or end part of a chemical name needs careful attention. Prefixes can be used to describe the ratio of the elements that are in the compound. This is used for non-metals. You should know the following prefixes: ‘mono’ (one), ’di’ (two) and ’tr’” (three).
Let’s look at some examples:
- [latex]\scriptsize \displaystyle \text{CO}[/latex] (carbon monoxide) – there is one atom of oxygen for every one atom of carbon
- [latex]\scriptsize \displaystyle \text{N}{{\text{O}}_{\text{2}}}[/latex] (nitrogen dioxide) – there are two atoms of oxygen for every one atom of nitrogen
- [latex]\scriptsize \displaystyle \text{S}{{\text{O}}_{\text{3}}}[/latex] (sulfur trioxide) – there are three atoms of oxygen for every one atom of sulfur.
There is a big difference between the ‘ide’, ‘ate’ and ‘ite’ suffixes used in chemical names
- As a general rule an ‘ide’ suffix indicates a non-metal element in a compound. e.g. sulfide [latex]\scriptsize \displaystyle {{\text{S}}^{{\text{-2}}}}[/latex], nitride [latex]\scriptsize \displaystyle {{\text{N}}^{{-3}}}[/latex] and phosphide [latex]\scriptsize \displaystyle {{\text{P}}^{{\text{-3}}}}[/latex]. The exceptions are hydroxide [latex]\scriptsize \displaystyle \text{O}{{\text{H}}^{\text{-}}}[/latex] and cyanide [latex]\scriptsize \displaystyle \text{C}{{\text{N}}^{\text{-}}}[/latex].
- The suffixes ‘ates’ and ‘ites’ always contain oxygen. e.g. nitrate [latex]\scriptsize \displaystyle \text{N}{{\text{O}}_{\text{3}}}^{\text{-}}[/latex] and nitrite [latex]\scriptsize \displaystyle \text{N}{{\text{O}}_{\text{2}}}^{\text{-}}[/latex].
- The suffix ‘ates’ always has a higher number of oxygen atoms than the corresponding ‘ites’. e.g. sulfate [latex]\scriptsize \displaystyle \text{SO}_{4}^{{-2}}[/latex] and sulfite [latex]\scriptsize \displaystyle \text{SO}_{3}^{{-2}}[/latex]
Polyatomic ions
Some ions are formed from groups of atoms. The formulae of compounds containing are worked out in a similar way to single atom ions, except when there is more than one polyatomic ion, then its formula is written inside brackets.
For example, calcium hydroxide contains [latex]\scriptsize \displaystyle \text{C}{{\text{a}}^{{\text{2+}}}}[/latex] and [latex]\scriptsize \displaystyle \text{O}{{\text{H}}^{-}}[/latex] ions. This is two positive charges and one negative charge. To balance, it will need, one [latex]\scriptsize \displaystyle \text{C}{{\text{a}}^{{\text{2+}}}}[/latex] ion and two [latex]\scriptsize \displaystyle \text{O}{{\text{H}}^{-}}[/latex] ions, so the formula is [latex]\scriptsize \displaystyle \text{Ca}{{\left( {\text{OH}} \right)}_{\text{2}}}[/latex].
Examples of common polyatomic ions are listed in table 1.
| Compound | Formula ion | Compound | Formula ion |
| Hydroxide | [latex]\scriptsize \displaystyle \text{O}{{\text{H}}^{-}}[/latex] | Phosphate | [latex]\scriptsize \text{PO}_{4}^{{-3}}[/latex] |
| Nitrite | [latex]\scriptsize \displaystyle \text{NO}_{2}^{{-1}}[/latex] | Hypochlorite | [latex]\scriptsize \displaystyle \text{Cl}{{\text{O}}^{\text{-}}}[/latex] |
| Nitrate | [latex]\scriptsize \text{NO}_{3}^{{-1}}[/latex] | Carbonate | [latex]\scriptsize \displaystyle \text{CO}_{3}^{{-2}}[/latex] |
| Hydrogen carbonate | [latex]\scriptsize \displaystyle \text{HCO}_{3}^{-}[/latex] | Chromate | [latex]\scriptsize \displaystyle \text{CrO}_{\text{4}}^{{\text{-2}}}[/latex] |
| Hydrogen sulfite | [latex]\scriptsize \displaystyle \text{HSO}_{3}^{-}[/latex] | Dichromate | [latex]\scriptsize \displaystyle \text{C}{{\text{r}}_{\text{2}}}\text{O}_{7}^{{-2}}[/latex] |
| Hydrogen sulfate | [latex]\scriptsize \displaystyle \text{HSO}_{4}^{-}[/latex] | Permanganate | [latex]\scriptsize \displaystyle \text{MnO}_{4}^{-}[/latex] |
| Sulfite | [latex]\scriptsize \displaystyle \text{SO}_{3}^{{-2}}[/latex] | Dihydrogen phosphate | [latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}[/latex] |
| Sulfate | [latex]\scriptsize \displaystyle \text{SO}_{4}^{{-2}}[/latex] | ||
| Thiosulfate | [latex]\scriptsize \displaystyle {{\text{S}}_{\text{2}}}\text{O}_{3}^{{-2}}[/latex] | Ammonium | [latex]\scriptsize \text{NH}_{4}^{+}[/latex] |
Chemical equations
A chemical equation is a written symbolic representation of a chemical reaction. The chemicals are given on the left-hand side of an arrow and the chemicals on the right-hand side.
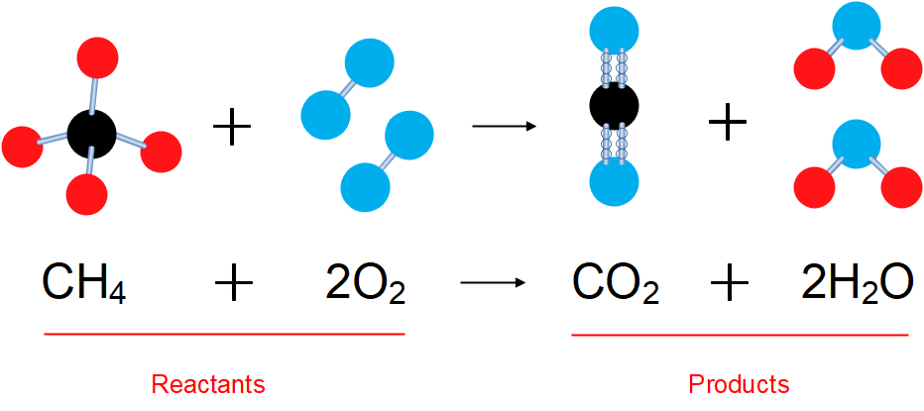
A chemical equation describes a chemical reaction by using symbols for the elements involved. The reaction in figure 1 shows methane ([latex]\scriptsize \text{C}{{\text{H}}_{4}}[/latex]) reacting with oxygen ([latex]\scriptsize {{\text{O}}_{2}}[/latex]) to form carbon dioxide ([latex]\scriptsize \text{C}{{\text{O}}_{2}}[/latex]) and water ([latex]\scriptsize {{\text{H}}_{\text{2}}}\text{O}[/latex]).
Written as a word equation, the reaction is: [latex]\scriptsize \text{methane + oxygen }\to \text{ carbon dioxide + water}[/latex].
To turn word equations into symbolic equations, we need to follow the given steps:
- Identify the reactants and products. This will help you know which symbols go on each side of the arrow and where the [latex]\scriptsize \displaystyle +[/latex] signs go.
- Write the correct formulae for all compounds.
- Sometimes a reactant or product is only an element (like hydrogen gas or oxygen gas). There are seven elements that are considered diatomic, meaning that, in their pure form, they are always found in pairs in nature (diatomic elements).
If we substitute chemical formulae into the equation from figure 1, it is written as:
[latex]\scriptsize \text{C}{{\text{H}}_{4}}+2{{\text{O}}_{2}}\to \text{C}{{\text{O}}_{2}}+2{{\text{H}}_{2}}\text{O}[/latex]
When sulfur dioxide burns in the presence of oxygen, sulfur trioxide is formed:
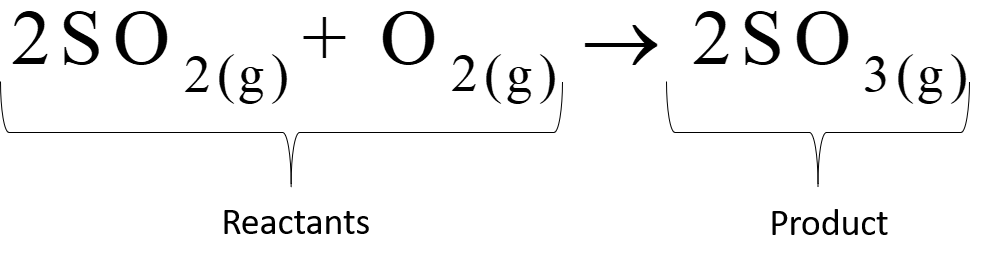
Table 2 shows the symbols used in chemical equations.
| Symbol | Description | Symbol | Description |
| + | used to separate multiple reactants or products | (s) | reactant or product in the solid state |
| → | arrow; separates reactants from products | (l) | reactant or product in the liquid state |
| ⇌ | replaces the arrow for reversible reactions | (g) | reactant or product in the gas state |
| (aq) | reactant or product in an aqueous solution (dissolved in water) |
Exercise 1.1
Transfer the following symbolic equations into word equations or word equations into symbolic equations.
- [latex]\scriptsize \displaystyle \text{HC}{{\text{l}}_{{\left( {\text{aq}} \right)}}}\text{+ NaO}{{\text{H}}_{{\left( {\text{aq}} \right)}}}\to \text{NaC}{{\text{l}}_{{\left( {\text{aq}} \right)}}}\text{+ }{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}[/latex]
- Gaseous propane, [latex]\scriptsize \displaystyle {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}[/latex], burns in oxygen gas to produce gaseous carbon dioxide and liquid water.
- Hydrogen fluoride gas ([latex]\scriptsize \displaystyle \text{HF}[/latex]) reacts with an aqueous solution of potassium carbonate ([latex]\scriptsize \displaystyle {{\text{K}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}[/latex]) to produce an aqueous solution of potassium fluoride, liquid water, and gaseous carbon dioxide.
- Hydrogen gas reacts with nitrogen gas to produce gaseous ammonia.
The full solutions can be found at the end of the unit.
Conservation of mass
The reaction shown in figure 1 is a combustion reaction where methane, the main ingredient in natural gas, is burnt. Count the atoms in the molecules. What do you notice about the number of red atoms (used to represent hydrogen) on the left and the right? You can do the same with the black atoms (carbon) and the blue atoms (oxygen).
In a chemical reaction the total mass of all the substances taking part in the reaction remains the same. The total number and kind of atoms in a reaction remains the same too. Mass cannot be created or destroyed in a chemical reaction.
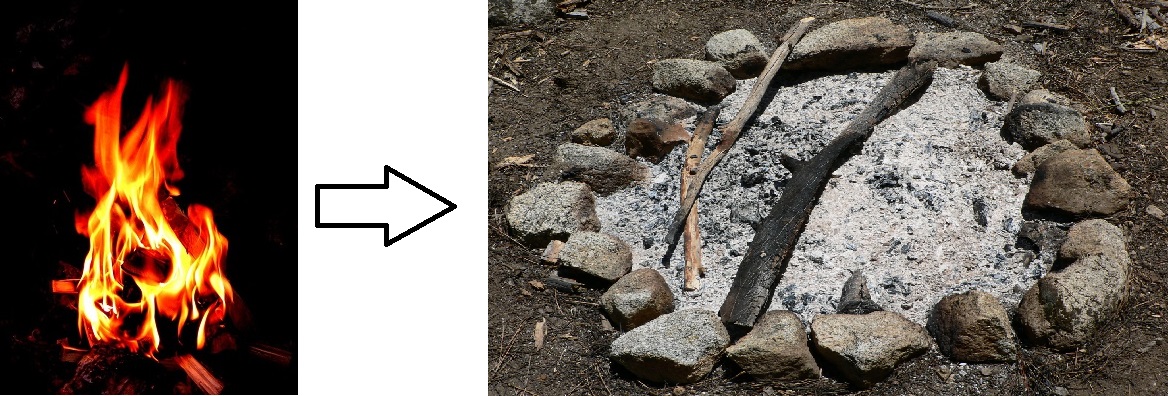
For example, when wood burns, the mass of the soot, ashes, and released gases equals the original mass of the charcoal and the oxygen when it first reacted. So the mass of the product equals the mass of the reactant. A reactant is the chemical reaction of two or more elements to make a new substance, and a product is the substance that is formed as the result of a chemical reaction. Atoms and their corresponding mass may not be created or destroyed but can be rearranged in different ratios to form other substances.
If you witness [latex]\scriptsize \displaystyle 10\text{ kg}[/latex] of wood burning, there are only ashes left after the burn, with a total mass of [latex]\scriptsize \displaystyle 1\text{ kg}[/latex], you may wonder where the other [latex]\scriptsize \displaystyle 9\text{ kg}[/latex] went. The other [latex]\scriptsize \displaystyle 9\text{ kg}[/latex] was released into the atmosphere as smoke and carbon dioxide, so the only thing left that you could see is the [latex]\scriptsize \displaystyle 1\text{ kg}[/latex] of ash. If you know the law of conservation of mass, then you know that the other [latex]\scriptsize \displaystyle 9\text{ kg}[/latex] has to go somewhere, because the total mass of the ash, smoke and released gases must equal the total mass of the tree and oxygen used for burning.
For any chemical equation (in a closed system) the mass of the reactants must be equal to the mass of the products. To make sure that this is the case, the number of atoms of each element in the reactants must be equal to the number of atoms of those same elements in the products. The law of conservation of mass says that no atoms can be made in a chemical reaction. Also, none can be destroyed.
Note
Watch this video on a demonstration of mass conservation by mulchem (Duration: 3.51).
Conservation of energy
When physical or chemical changes occur, they are generally accompanied by a transfer of energy. The law of conservation of energy states that in any physical or chemical process, energy is neither created nor destroyed. In other words, the entire energy in the universe is conserved. To better understand the energy changes taking place during a reaction, we need to define two parts of the universe, called the system and the surroundings.
The surroundings is everything in the universe that is not part of the system. In practical terms for a laboratory chemist, the system is the chemicals being reacted, while the surroundings is the immediate vicinity within the room. During most processes, energy is exchanged between the system and the surroundings.
During an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Because the surroundings is gaining heat from the system, the temperature of the surroundings increases. Exothermic and endothermic reactions can be thought of as having energy as either a product of the reaction or a reactant. Exothermic reactions give off energy, so energy is a product. Endothermic reactions require energy, so energy is a reactant.
Phase changes are also classified in a similar way. The change from gas to liquid (condensation) and liquid to solid (freezing) are exothermic. The change from solid to liquid (melting), and liquid to gas (evaporation and boiling) are endothermic. The exothermic processes release heat to the surroundings while the endothermic processes absorb heat from the surroundings.
When bonds form, energy is released, and the principle of conservation of energy demands that the opposite is true: that breaking bonds requires energy. Atoms bond together to form compounds because in doing so they attain lower energies than they possess as individual atoms. A quantity of energy, equal to the difference between the energies of the bonded atoms and the energies of the separated atoms, is released, usually as heat. That is, the bonded atoms have a lower energy than the individual atoms do. When atoms combine to make a compound, energy is always given off, and the compound has a lower overall energy.
When a chemical reaction occurs, molecular bonds are broken, and other bonds are formed to make different molecules. For example, the bonds of two water molecules are broken to form hydrogen and oxygen.
Energy is always required to break a bond, which is known as bond energy. While the concept may seem simple, bond energy serves a very important purpose in describing the structure and characteristics of a molecule.
When a chemical reaction occurs, the atoms in the reactants rearrange their chemical bonds to make products. The new arrangement of bonds does not have the same total energy as the bonds in the reactants. Therefore, when chemical reactions occur, there will always be an accompanying energy change. In this process, one adds energy to the reaction to break bonds, and extracts energy for the bonds that are formed. Energy is always required to break a bond. Energy is released when a bond is made.
Balancing chemical equations
A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. We can write a skeleton chemical equation for the reaction of carbon with hydrogen gas to form methane [latex]\scriptsize \text{C}{{\text{H}}_{\text{4}}}[/latex]:
[latex]\scriptsize \displaystyle {{\text{C}}_{{\left( \text{s} \right)}}}+{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\to \text{C}{{\text{H}}_{{\text{4}\left( \text{g} \right)}}}[/latex]
Remember that hydrogen is a diatomic molecule and so is written as [latex]\scriptsize {{\text{H}}_{\text{2}}}[/latex].
When we count the number of atoms of both elements, we see that the equation is not balanced. There are only [latex]\scriptsize 2[/latex] atoms of hydrogen on the reactant side of the equation, while there are [latex]\scriptsize \displaystyle 4[/latex] atoms of hydrogen on the product side. The subscript in the formula [latex]\scriptsize {{\text{H}}_{2}}[/latex] cannot be changed as it represents the ratio in which hydrogen atoms join to form a molecule. Therefore the only way to balance the above equation is by adding a of [latex]\scriptsize \displaystyle 2[/latex] in front of the formula for hydrogen:
[latex]\scriptsize \displaystyle {{\text{C}}_{{\left( \text{s} \right)}}}\text{+ 2}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\to \text{C}{{\text{H}}_{{\text{4}\left( \text{g} \right)}}}[/latex]
A coefficient is a small whole number placed in front of a formula in an equation in order to balance it. The [latex]\scriptsize \displaystyle 2[/latex] in front of the [latex]\scriptsize {{\text{H}}_{\text{2}}}[/latex] means that there are a total of [latex]\scriptsize \displaystyle 2\times 2=4~[/latex] atoms of hydrogen as reactants (or [latex]\scriptsize \displaystyle 2[/latex] molecules of [latex]\scriptsize {{\text{H}}_{2}}[/latex]).
Let’s look at another example.
Example 1.1
Aqueous solutions of lead (II) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead (II) chloride. Write the balanced chemical equation for this reaction.
Solution
Step 1: Write the skeleton equation for the reaction
[latex]\scriptsize \displaystyle \text{Pb}{{\left( {\text{N}{{\text{O}}_{\text{3}}}} \right)}_{{\text{2}\left( {\text{aq}} \right)}}}\text{+ NaC}{{\text{l}}_{{\left( {\text{aq}} \right)}}}\to \text{NaN}{{\text{O}}_{{\text{3}\left( {\text{aq}} \right)}}}\text{+ PbC}{{\text{l}}_{{\text{2}\left( \text{s} \right)}}}[/latex]
Step 2: Count the number of each atom or polyatomic ion on both sides of the equation
Reactants:
[latex]\scriptsize \text{1 Pb}[/latex] atom, [latex]\scriptsize \displaystyle 2\text{N}{{\text{O}}^{{-3}}}[/latex] ions, [latex]\scriptsize \displaystyle 1\text{ Na}[/latex] atom, [latex]\scriptsize \displaystyle \text{1 Cl}[/latex] atom
Products:
[latex]\scriptsize \text{1 Pb}[/latex] atom, [latex]\scriptsize \displaystyle \text{1N}{{\text{O}}^{{-3}}}[/latex] ions, [latex]\scriptsize \displaystyle 1\text{ Na}[/latex] atom, [latex]\scriptsize \displaystyle \text{2 Cl}[/latex] atoms
The nitrate ions and the chlorine atoms are unbalanced. If we start by placing a [latex]\scriptsize \displaystyle 2[/latex] in front of the[latex]\scriptsize \displaystyle \text{NaCl}[/latex], this increases the reactant counts to [latex]\scriptsize \displaystyle 2\text{ Na}[/latex] atoms and [latex]\scriptsize \displaystyle 2\text{ Cl}[/latex] atoms.
So then place a [latex]\scriptsize \displaystyle 2[/latex] in front of the [latex]\scriptsize \displaystyle \text{NaN}{{\text{O}}_{\text{3}}}[/latex]. The result is:
[latex]\scriptsize \displaystyle \text{Pb}{{\left( {\text{N}{{\text{O}}_{\text{3}}}} \right)}_{{\text{2}\left( {\text{aq}} \right)}}}\text{+2NaC}{{\text{l}}_{{\left( {\text{aq}} \right)}}}\to \text{2NaN}{{\text{O}}_{{\text{3}\left( {\text{aq}} \right)}}}\text{+PbC}{{\text{l}}_{{\text{2}\left( \text{s} \right)}}}[/latex]
If we now count the atoms and polyatomic ions on both sides of the equation, we can see the equation is now balanced.
Take note!
These are the basic techniques for balancing equations:
- Identify reactants and products and write an unbalanced word equation.
- Determine the correct chemical formulas for each reactant and product (using valency rules), write the reactants left of the arrow and the products right of the arrow (the order is not important).
- Count the number of atoms of each element that appears as a reactant and as a product. If a polyatomic ion is unchanged on both sides of the equation, count it as a unit.
- Leave a space for coefficients. Placing a line in front of each chemical is a good way to do this: __Reactant(A) + __Reactant(B) → __Product(X) + __Product(Y)
- Balance each element one at a time by placing coefficients in front of the formulae (use a pencil as you may have to go back and change your workings). No coefficient is written for a [latex]\scriptsize \displaystyle 1[/latex]. It is best to begin by balancing elements that only appear in one chemical formula on each side of the equation. NEVER change the subscripts in a chemical formula – you can only balance equations by using coefficients.
- Check each atom or polyatomic ion to be sure that they are equal on both sides of the equation.
- Make sure that all coefficients are in the lowest possible ratio. If necessary, reduce to the lowest ratio, then add phase symbols.
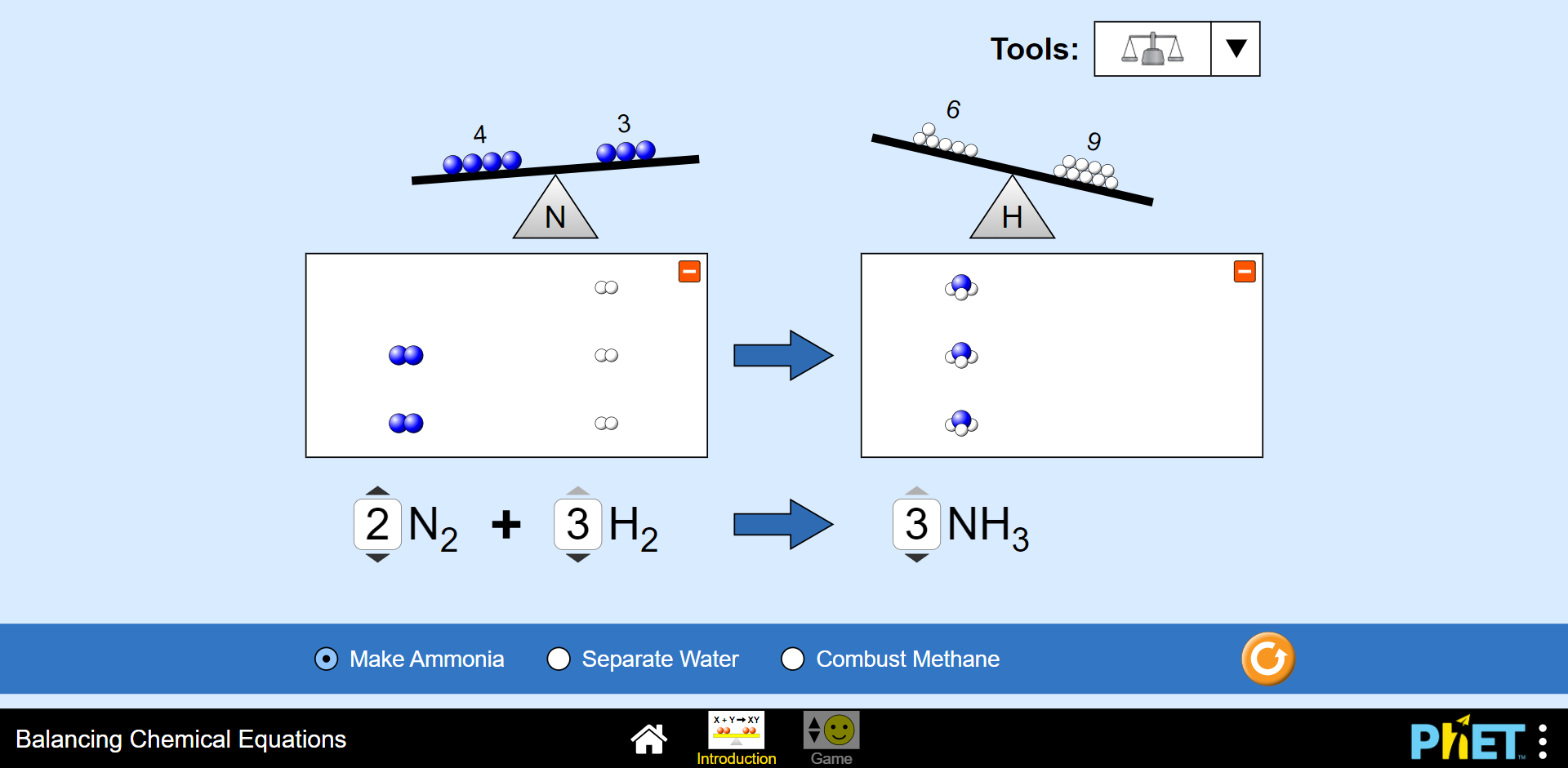
Activity 1: Balance chemical equations
Time required: 10 minutes
What you need:
- internet access
What to do:
- Go to this online simulation.
- Click on Introduction.
- Press the tools button and add the scales.
- Balance the ammonia by adding coefficients in front of nitrogen, hydrogen and ammonia until the equation is balanced.
- Repeat for water, and methane.
What did you find?
By increasing the coefficients on both sides of the equation, you can determine the balanced equation.
Example 1.2
Balance the following equation:
[latex]\scriptsize \text{Mg+HCl}\to \text{MgC}{{\text{l}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}[/latex]
Solution
Step 1: Identify the reactants and products
The reactants are magnesium (Mg) and hydrogen chloride (HCl)
The products are Magnesium chloride (MgCl2) and Hydrogen (H2)
Step 2: Write the skeleton equation for the reaction
[latex]\scriptsize \text{Mg+HCl}\to \text{MgC}{{\text{l}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}[/latex]
Step 3: Count the number of atoms of each element in the reactants and products
Reactants: Mg = [latex]\scriptsize \displaystyle 1[/latex] atom, H = [latex]\scriptsize \displaystyle 1[/latex] atom, Cl = [latex]\scriptsize \displaystyle 1[/latex] atom
Products: Mg =[latex]\scriptsize \displaystyle 1[/latex] atom, H = [latex]\scriptsize 2[/latex] atoms, Cl = [latex]\scriptsize 2[/latex] atoms
Step 4: Balance the equation
The equation is not balanced since there are two chlorine atoms in the product and only one in the reactants. If we add a coefficient of two to the HCl to increase the number of H and Cl atoms in the reactants, the equation will look like this:
[latex]\scriptsize \text{Mg+2HCl}\to \text{MgC}{{\text{l}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}[/latex]
Step 5: Check that the atoms are balanced
If we count the atoms on each side of the equation, we find the following:
Reactants: Mg = [latex]\scriptsize \displaystyle 1[/latex] atom, H = [latex]\scriptsize 2[/latex] atoms, Cl = [latex]\scriptsize 2[/latex]atoms
Products: Mg = [latex]\scriptsize \displaystyle 1[/latex] atom, H = [latex]\scriptsize 2[/latex] atoms, Cl = [latex]\scriptsize 2[/latex] atoms
The equation is balanced. The final equation is: [latex]\scriptsize \text{Mg+2HCl}\to \text{MgC}{{\text{l}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}[/latex]
Example 1.3
Balance the following equation: [latex]\scriptsize \text{C}{{\text{H}}_{\text{4}}}\text{+}{{\text{O}}_{\text{2}}}\to \text{C}{{\text{O}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Solution
Step 1: Count the number of atoms of each element in the reactants and products
Reactants: [latex]\scriptsize \displaystyle \text{C = 1, H = 4, O = 2}[/latex]
Products: [latex]\scriptsize \displaystyle \text{C = 1, H = 2, O = 3}[/latex]
Step 2: Balance the equation
If we add a coefficient of [latex]\scriptsize \displaystyle 2[/latex] to [latex]\scriptsize {{\text{H}}_{\text{2}}}\text{O}[/latex], then the number of hydrogen atoms in the products will be [latex]\scriptsize \displaystyle 4[/latex], which is the same as for the reactants. So the equation will be:
[latex]\scriptsize \text{C}{{\text{H}}_{\text{4}}}\text{+}{{\text{O}}_{\text{2}}}\to \text{C}{{\text{O}}_{\text{2}}}\text{+2}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Step 3: Check that the atoms balance
Reactants: C = [latex]\scriptsize \displaystyle 1[/latex], H = [latex]\scriptsize 4[/latex], O =[latex]\scriptsize 2[/latex]
Products: C = [latex]\scriptsize \displaystyle 1[/latex], H = [latex]\scriptsize 4[/latex], O = [latex]\scriptsize 4[/latex]
You will see that, although the number of hydrogen atoms now balances, there are more oxygen atoms in the products. You now need to repeat the previous step. If we put a coefficient of [latex]\scriptsize \displaystyle 2[/latex] in front of [latex]\scriptsize \displaystyle {{\text{O}}_{2}}[/latex], then we will increase the number of oxygen atoms in the reactants by [latex]\scriptsize \displaystyle 2[/latex]. The new equation is:
[latex]\scriptsize \text{C}{{\text{H}}_{\text{4}}}\text{+2}{{\text{O}}_{\text{2}}}\to \text{C}{{\text{O}}_{\text{2}}}\text{+2}{{\text{H}}_{\text{2}}}\text{O}[/latex]
When we check the number of atoms again, we find that the number of atoms of each element in the reactants is the same as the number in the products. The equation is now balanced.
Example 1.4
In our bodies, sugar ([latex]\scriptsize \displaystyle {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}[/latex]) reacts with the oxygen we breathe in to produce carbon dioxide, water, and energy. Write the balanced equation for this reaction.
Solution
Step 1: Identify the reactants and products in the reaction
Reactants: sugar ([latex]\scriptsize \displaystyle {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}[/latex]) and oxygen ([latex]\scriptsize \displaystyle {{\text{O}}_{2}}[/latex])
Products: carbon dioxide ([latex]\scriptsize \displaystyle \text{C}{{\text{O}}_{\text{2}}}[/latex]) and water ([latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}\text{O}[/latex])
Step 2: Write the skeleton equation
[latex]\scriptsize {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\text{+}{{\text{O}}_{\text{2}}}\to \text{C}{{\text{O}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Step 3: Count the number of atoms of each element in the reactants and in the products
Reactants: [latex]\scriptsize \displaystyle \text{C = 6, H = 12, O = 8}[/latex]
Products: [latex]\scriptsize \displaystyle \text{C = 1, H = 2, O = 3}[/latex]
Step 4: Balance the equation
It is easier to start with carbon as it only appears once on each side. If we add a 6 in front of CO2, the equation looks like this: [latex]\scriptsize {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\text{+}{{\text{O}}_{\text{2}}}\to 6\text{C}{{\text{O}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Reactants: [latex]\scriptsize \displaystyle \text{C = 6, H = 12, O = 8}[/latex]
Products: [latex]\scriptsize \displaystyle \text{C = 6, H = 2, O = 13}[/latex]
Change the coefficients again to try to balance the equation.
Let us try to get the number of hydrogens the same this time.
[latex]\scriptsize {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\text{+}{{\text{O}}_{\text{2}}}\to 6\text{C}{{\text{O}}_{\text{2}}}\text{+6}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Reactants: [latex]\scriptsize \displaystyle \text{C = 6, H = 12, O = 8}[/latex]
Products: [latex]\scriptsize \displaystyle \text{C = 6, H = 12, O = 18}[/latex]
Now we just need to balance the oxygen atoms: [latex]\scriptsize {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\text{+6}{{\text{O}}_{\text{2}}}\to 6\text{C}{{\text{O}}_{\text{2}}}\text{+6}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Reactants: [latex]\scriptsize \displaystyle \text{C = 6, H = 12, O = 18}[/latex]
Products: [latex]\scriptsize \displaystyle \text{C = 6, H = 12, O = 18}[/latex]
Exercise 1.2
- Balance the following equations:
- [latex]\scriptsize \text{Mg+}{{\text{O}}_{\text{2}}}\to \text{MgO}[/latex]
- [latex]\scriptsize \displaystyle \text{CaC}{{\text{l}}_{\text{2}}}\text{+N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\to \text{CaC}{{\text{O}}_{\text{3}}}\text{+NaCl}[/latex]
- [latex]\scriptsize \displaystyle {{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{{\text{11}}}}\text{+}{{\text{O}}_{\text{2}}}\to \text{C}{{\text{O}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}\text{O}[/latex]
- Propane is a fuel that is commonly used as a heat source for engines and homes. Balance the following equation for the combustion of propane:
[latex]\scriptsize {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}_{{\text{(l)}}}\text{+}{{\text{O}}_{{\text{2(g)}}}}\to \text{C}{{\text{O}}_{{\text{2(g)}}}}\text{+H}{}_{\text{2}}{{\text{O}}_{{\text{(g)}}}}[/latex]
The full solutions can be found at the end of the unit.
Summary
In this unit you have learnt the following:
- A chemical reaction is the process by which one or more substances are changed into one or more new substances.
- Chemical reactions are represented by chemical equations.
- Chemical equations have reactants on the left, an arrow, and the products on the right. A chemical equation uses symbols to describe a chemical reaction.
- The law of conservation of mass states that the mass of a closed system of substances will remain constant, regardless of the processes acting inside the system. Matter can change form but cannot be created or destroyed.
- In any chemical reaction, the law of conservation of mass applies. This also means that the total number of atoms in the reactants must be the same as the total number of atoms in the product.
- If the number of atoms of each element in the reactants is the same as the number of atoms of each element in the product, then the equation is balanced.
- If the number of atoms of each element in the reactants is not the same as the number of atoms of each element in the product, then the equation is not balanced.
- Chemical processes are labelled as exothermic or endothermic based on whether they give off or absorb energy, respectively.
- Atoms are held together by a certain amount of energy called bond energy.
- Energy is released to generate bonds, which is why the enthalpy change for breaking bonds is positive. Energy is required to break bonds. Atoms are much happier when they are ‘married’ and release energy because it is easier and more stable to be in a relationship (
- To balance an equation, coefficients can be placed in front of the reactants and products until the number of atoms of each element is the same on both sides of the equation.
- The state of the compounds in a chemical reaction can be expressed in the chemical equation by using one of four symbols. The symbols are g (gas), l (liquid), s (solid) and aq (aqueous solutions). These symbols are written in brackets after the compound.
Unit 1: Assessment
Suggested time to complete: 30 minutes
- Balance the following equations:
- [latex]\scriptsize \displaystyle \text{Fe + C}{{\text{l}}_{\text{2}}}\text{ }\to \text{ FeC}{{\text{l}}_{\text{3}}}[/latex]
- [latex]\scriptsize \displaystyle \text{FeB}{{\text{r}}_{\text{3}}}\text{ + }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }\to \text{ F}{{\text{e}}_{\text{2}}}{{\left( {\text{S}{{\text{O}}_{\text{4}}}} \right)}_{\text{3}}}\text{ + HBr}[/latex]
- [latex]\scriptsize \displaystyle {{\text{C}}_{\text{4}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{{\text{3 }}}}\text{+ }{{\text{H}}_{\text{2}}}\text{O }\to \text{ }{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}[/latex]
- [latex]\scriptsize \displaystyle {{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\text{O + }{{\text{O}}_{\text{2}}}\text{ }\to \text{C}{{\text{O}}_{\text{2}}}\text{ + }{{\text{H}}_{\text{2}}}\text{O}[/latex]
- [latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}\text{SiC}{{\text{l}}_{\text{2}}}\text{ + }{{\text{H}}_{\text{2}}}\text{O}\to \text{ }{{\text{H}}_{\text{8}}}\text{S}{{\text{i}}_{\text{4}}}{{\text{O}}_{\text{4}}}\text{ + HCl}[/latex]
- [latex]\scriptsize \displaystyle {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{{\text{2 }}}}\text{+ NaH+ HCl }\to \text{ }{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{2}}}\text{ + NaCl}[/latex]
- Sulfur can be produced by the Claus process. This two-step process involves reacting hydrogen sulfide with oxygen and then reacting the sulfur dioxide that is produced with more hydrogen sulfide. The equations for these two reactions are:
[latex]\scriptsize \displaystyle \begin{align*}&{{\text{H}}_{\text{2}}}\text{S + }{{\text{O}}_{\text{2}}}\text{ }\to \text{S}{{\text{O}}_{\text{2}}}\text{+ }{{\text{H}}_{\text{2}}}\text{O}\\&{{\text{H}}_{\text{2}}}\text{S + S}{{\text{O}}_{\text{2}}}\text{ }\to \text{S + }{{\text{H}}_{\text{2}}}\text{O}\end{align*}[/latex]
Balance these two equations. - Hydrogen fuel cells are extremely important in the development of alternative energy sources. Many of these cells work by reacting hydrogen and oxygen gases together to form water, a reaction which also produces electricity. Balance the following equation:
[latex]\scriptsize {{\text{H}}_{{\text{2(g)}}}}+{{\text{O}}_{{\text{2(g)}}}}\to {{\text{H}}_{\text{2}}}{{\text{O}}_{{\text{(l)}}}}[/latex] - Aspartame, an artificial sweetener, has the formula [latex]\scriptsize \displaystyle {{\text{C}}_{{\text{14}}}}{{\text{H}}_{{\text{18}}}}{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}[/latex]. Write the balanced equation for its combustion (reaction with oxygen) to form carbon dioxide gas, liquid water, and nitrogen gas.
The full solutions can be found at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- An aqueous solution of hydrochloric acid reacts with an aqueous solution of sodium hydroxide to produce an aqueous solution of sodium chloride and liquid water:
[latex]\scriptsize \displaystyle \text{hydrochloric aci}{{\text{d}}_{{\text{(aq)}}}}\text{+ sodium hydroxid}{{\text{e}}_{{\text{(aq)}}}}\text{ }\!\!~\!\!\text{ }\to \text{ }\!\!~\!\!\text{ sodium chlorid}{{\text{e}}_{{\text{(aq)}}}}\text{+ wate}{{\text{r}}_{{\text{(l)}}}}[/latex] - Reactants: propane ([latex]\scriptsize {{\text{C}}_{3}}{{\text{H}}_{\text{8}}}[/latex]) and oxygen ([latex]\scriptsize \displaystyle {{\text{O}}_{2}}[/latex])
Product: carbon dioxide ([latex]\scriptsize \text{C}{{\text{O}}_{\text{2}}}[/latex]) and water ([latex]\scriptsize {{\text{H}}_{\text{2}}}\text{O}[/latex])
[latex]\scriptsize \displaystyle {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}_{{\left( \text{g} \right)}}\text{+ }{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{+ }{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}[/latex] - Reactants: hydrogen fluoride and potassium carbonate
Products: potassium fluoride, water, and carbon dioxide
[latex]\scriptsize \displaystyle \text{H}{{\text{F}}_{{\left( \text{g} \right)}}}\text{+ }{{\text{K}}_{\text{2}}}\text{C}{{\text{O}}_{{\text{3}\left( {\text{aq}} \right)}}}\to \text{K}{{\text{F}}_{{\left( {\text{aq}} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\text{+C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex] - d. [latex]\scriptsize \displaystyle {{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{+ }{{\text{N}}_{{\text{2}\left( \text{g} \right)}}}\to \text{N}{{\text{H}}_{{\text{3}\left( \text{g} \right)}}}[/latex]
Exercise 1.2
- .
- The oxygen needs balancing: [latex]\scriptsize \text{2Mg+}{{\text{O}}_{\text{2}}}\to 2\text{MgO}[/latex]
- The sodium and chlorine need balancing: [latex]\scriptsize \displaystyle \text{CaC}{{\text{l}}_{\text{2}}}\text{+N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\to \text{CaC}{{\text{O}}_{\text{3}}}\text{+2NaCl}[/latex]
- Balance the carbon: [latex]\scriptsize \displaystyle {{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{{\text{11}}}}\text{+}{{\text{O}}_{\text{2}}}\to 12\text{C}{{\text{O}}_{\text{2}}}\text{+}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Balance the hydrogen: [latex]\scriptsize \displaystyle {{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{{\text{11}}}}\text{+}{{\text{O}}_{\text{2}}}\to 12\text{C}{{\text{O}}_{\text{2}}}\text{+11}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Balance the oxygen: [latex]\scriptsize \displaystyle {{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{{\text{11}}}}\text{+12}{{\text{O}}_{\text{2}}}\to 12\text{C}{{\text{O}}_{\text{2}}}\text{+11}{{\text{H}}_{\text{2}}}\text{O}[/latex]
- Balance the hydrogen: [latex]\scriptsize {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}_{{\text{(l)}}}\text{+}{{\text{O}}_{{\text{2(g)}}}}\to \text{C}{{\text{O}}_{{\text{2(g)}}}}\text{+4H}{}_{\text{2}}{{\text{O}}_{{\text{(g)}}}}[/latex]
Balance the carbon: [latex]\scriptsize {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}_{{\text{(l)}}}\text{+}{{\text{O}}_{{\text{2(g)}}}}\to 3\text{C}{{\text{O}}_{{\text{2(g)}}}}\text{+4H}{}_{\text{2}}{{\text{O}}_{{\text{(g)}}}}[/latex]
Balance the oxygen: [latex]\scriptsize {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}_{{\text{(l)}}}\text{+5}{{\text{O}}_{{\text{2(g)}}}}\to 3\text{C}{{\text{O}}_{{\text{2(g)}}}}\text{+4H}{}_{\text{2}}{{\text{O}}_{{\text{(g)}}}}[/latex]
Balanced equation: [latex]\scriptsize {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}_{{\text{(l)}}}\text{+5}{{\text{O}}_{{\text{2(g)}}}}\to 3\text{C}{{\text{O}}_{{\text{2(g)}}}}\text{+4H}{}_{\text{2}}{{\text{O}}_{{\text{(g)}}}}[/latex]
Unit 1: Assessment
- .
- [latex]\scriptsize \displaystyle \text{2 Fe + 3 C}{{\text{l}}_{\text{2}}}\text{ }\to \text{ 2 FeC}{{\text{l}}_{\text{3}}}[/latex]
- [latex]\scriptsize \displaystyle \text{2FeB}{{\text{r}}_{\text{3}}}\text{ + 3}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }\to \text{ F}{{\text{e}}_{\text{2}}}{{\left( {\text{S}{{\text{O}}_{\text{4}}}} \right)}_{\text{3}}}\text{ + 6HBr}[/latex]
- [latex]\scriptsize \displaystyle {{\text{C}}_{\text{4}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{{\text{3 }}}}\text{+ }{{\text{H}}_{\text{2}}}\text{O }\to \text{ 2}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}[/latex]
- [latex]\scriptsize \displaystyle {{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\text{O + 6}{{\text{O}}_{\text{2}}}\text{ }\to 4\text{C}{{\text{O}}_{\text{2}}}\text{ + 5}{{\text{H}}_{\text{2}}}\text{O}[/latex]
- [latex]\scriptsize \displaystyle \text{4}{{\text{H}}_{\text{2}}}\text{SiC}{{\text{l}}_{\text{2}}}\text{ + 4}{{\text{H}}_{\text{2}}}\text{O}\to \text{ }{{\text{H}}_{\text{8}}}\text{S}{{\text{i}}_{\text{4}}}{{\text{O}}_{\text{4}}}\text{ + 8HCl}[/latex]
- [latex]\scriptsize \displaystyle {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{{\text{2 }}}}\text{+ 2NaH + 2HCl }\to \text{ }{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{2}}}\text{ + 2NaCl}[/latex]
- [latex]\scriptsize \displaystyle \text{2}{{\text{H}}_{\text{2}}}\text{S+3}{{\text{O}}_{\text{2}}}\to 2\text{S}{{\text{O}}_{\text{2}}}\text{+2}{{\text{H}}_{\text{2}}}\text{O}[/latex]
[latex]\scriptsize \displaystyle \text{4}{{\text{H}}_{\text{2}}}\text{S+2S}{{\text{O}}_{\text{2}}}\to 6\text{S+4}{{\text{H}}_{\text{2}}}\text{O}[/latex] - [latex]\scriptsize \text{2}{{\text{H}}_{{\text{2(g)}}}}+{{\text{O}}_{{\text{2(g)}}}}\to 2{{\text{H}}_{\text{2}}}{{\text{O}}_{{\text{(l)}}}}[/latex]
- [latex]\scriptsize \displaystyle {{\text{C}}_{{\text{14}}}}{{\text{H}}_{{\text{18}}}}{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}_{{\text{(s)}}}+16{{\text{O}}_{\text{2}}}_{{\text{(g)}}}\to 14\text{C}{{\text{O}}_{{\text{2(g)}}}}\text{+ 9}{{\text{H}}_{\text{2}}}\text{O + }{{\text{N}}_{{\text{2(g)}}}}[/latex]
Media Attributions
- Fig 1 © DHET is licensed under a CC BY (Attribution) license
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
- Fig 3 © LibreTextx is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 4 © DHET is licensed under a CC BY (Attribution) license
- Fig 5 © DHET is licensed under a CC BY (Attribution) license
notations that give the type and ratio of the atoms that make up a particular chemical compound
ion composed of two or more atoms
the starting materials in a chemical reaction
the substance that is formed as the result of a chemical reaction
the number in front of the formula; the coefficient tells us how many molecules of a given formula are present
