Mechanical Properties of matter: State, analyse and apply principles of atomic combinations: molecular structure
Unit 3: Oxidation numbers
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Calculate oxidation number of atoms in molecules to explain their relative richness in electrons.
What you should know:
Before you start this unit, make sure you can:
- Summarise the principles of chemical reactions. Refer to level 2 subject outcome 6.3 unit 1 to revise this.
- Explain molecules and molecular structure. Refer to level 3 subject outcome 5.2 unit 1 to revise this.
- Describe electronegativity and polarity. Refer to level 3 subject outcome 5.2 unit 2 to revise this.
Introduction
Parts of the text in this unit were sourced from Siyavula Physical Science Gr 11 Learner’s Book, Chapter 3, released under a CC-BY licence.
In this unit you will learn about how oxidation numbers are used to show how many electrons are lost or gained by an atom during the formation of a compound in a redox reaction.
Oxidation-reduction reactions
– (redox) reactions are a common type of reaction where one substance loses electrons (called oxidation) and another substance gains electrons (called reduction). Two further terms used in redox reactions are and . An element that is oxidised is called a reducing agent, while an element that is reduced is called an oxidising agent.
Examples of redox reactions include rusting, photosynthesis, combustion, and respiration.
involve the exchange of electrons. One loses electrons and becomes positively charged, while the other species gains electrons and becomes negatively charged. In a redox reaction the charges on the atoms involved will change because of the transfer of electrons. The more electronegative atom will gain electrons and the atom with the lower electronegativity will lose electrons. In the reaction between lithium and fluorine, the electronegativity of lithium is [latex]\scriptsize 1.0[/latex] and the electronegativity of fluorine is [latex]\scriptsize 4.0[/latex]. This means that lithium will readily lose an electron and fluorine will readily accept an electron. The lithium will be oxidised (it will lose its [latex]\scriptsize \displaystyle 1[/latex] valence electron, and fluorine will be reduced (it will gain the electron from lithium). Because the lithium has lost an electron it will become an ion with a charge of [latex]\scriptsize \displaystyle {{1}^{+}}[/latex]. The fluorine atom having gained an electron will become an ion with a charge of [latex]\scriptsize \displaystyle {{1}^{-}}[/latex].
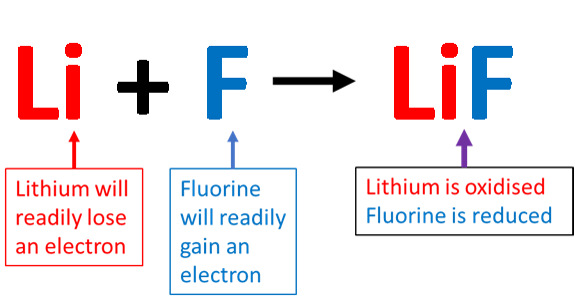
The reaction stages are called :
[latex]\scriptsize \text{Li }\to \text{L}{{\text{i}}^{+}}+{{e}^{-}}[/latex] oxidation half reaction
[latex]\scriptsize \text{F + }{{\text{e}}^{-}}\to {{\text{F}}^{-}}[/latex] reduction half reaction
The lithium atom starts with a neutral charge, then loses an electron to become an ion with a charge of [latex]\scriptsize \displaystyle {{1}^{+}}[/latex]. The fluorine atom starts with a neutral charge, then gains an electron to become an ion with a charge of [latex]\scriptsize \displaystyle {{1}^{-}}[/latex].
The number of electrons lost or gained is determined by the valency of the element. In the overall redox reaction, the total number of electrons lost must be equal to the total number of electrons gained.
In another example, sodium metal is oxidised to form sodium oxide. The balanced equation for this is:
[latex]\scriptsize \displaystyle \text{4Na+}{{\text{O}}_{2}}\to 2\text{N}{{\text{a}}_{\text{2}}}\text{O}[/latex]
In the above reaction sodium and oxygen are both neutral and so have no charge.
If we look at the half reactions:
[latex]\scriptsize \text{2Na}\to 2\text{N}{{\text{a}}^{+}}+2{{e}^{-}}[/latex] the sodium is oxidised – it has lost electrons
[latex]\scriptsize \text{O + 2}{{\text{e}}^{-}}\to {{\text{O}}^{{2-}}}[/latex] the oxygen is reduced – it has gained electrons
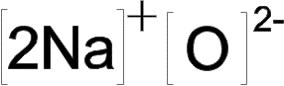
Because an oxygen atom has a valency of [latex]\scriptsize \displaystyle 2[/latex], it will gain two electrons to become an ion with a charge of[latex]\scriptsize \displaystyle {{2}^{-}}[/latex]. Sodium has a valency of [latex]\scriptsize \displaystyle 1[/latex], so it can only lose one electron. Therefore two sodium atoms need to each donate one electron to balance the overall electron transfer.
Rust occurs naturally when iron reacts with oxygen in the air to form iron oxide. Oxygen is a very good oxidizing agent whereas iron is a reducing agent. Therefore, the iron atom readily gives up electrons when exposed to oxygen. The chemical formula for iron oxide is [latex]\scriptsize \text{F}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}[/latex]. This process will happen faster when water is involved:
First iron is oxidised in water to form iron ions:
[latex]\scriptsize \text{2Fe}\to 2\text{F}{{\text{e}}^{{2+}}}+\text{ }4\text{e}-[/latex]
Then the oxygen which is dissolved in water is reduced and hydroxide ions form:
[latex]\scriptsize {{\text{O}}_{\text{2}}}\text{+2}{{\text{H}}_{\text{2}}}\text{O}+4\text{e}-\to 4\text{O}{{\text{H}}^{-}}[/latex]
Next the iron ion and the hydroxide ion react to form iron hydroxide:
[latex]\scriptsize \text{2F}{{\text{e}}^{{\text{2+}}}}\text{+4O}{{\text{H}}^{\text{-}}}\to \text{2Fe(OH}{{\text{)}}_{\text{2}}}[/latex]
Then iron oxide reacts with oxygen to yield red rust:
[latex]\scriptsize \text{F}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{.}{{\text{H}}_{\text{2}}}\text{O}[/latex]
Note: It is important to remember that these reactions happen simultaneously.

There is an easy mnemonic for remembering reduction and oxidation, OIL RIG:
| O | Oxidation |
| I | Is |
| L | Loss |
| R | Reduction |
| I | Is |
| G | Gain |
Exercise 3.1
Choose the correct answer for each question.
- Which change is oxidation?
- gain of electrons
- gain of hydrogen
- loss of oxygen
- loss of electrons
- Oxidation and reduction occur:
- simultaneously.
- in separate reactions.
- on the product side of the reaction.
- The species that is being reduced will have:
- a more negative charge after the reduction has occurred.
- a more positive charge after the reduction has occurred.
- no change in its charge after the reduction has occurred.
- Simply stated, the gain of electrons is:
- reduction
- oxidation
- sublimation
- A reducing agent is:
- the species which is gaining electrons.
- the species which is losing electrons.
- the latest anti-hero in a Marvel movie.
The full solutions can be found at the end of the unit.
Oxidation numbers
An is the charge an atom would have if it were in a compound composed of ions. By giving elements an oxidation number, it is possible to keep track of whether that element is losing or gaining electrons during a chemical reaction and whether given reactants are oxidised or reduced in a reaction. The loss of electrons in one part of the reaction must be balanced by a gain of electrons in another part of the reaction.
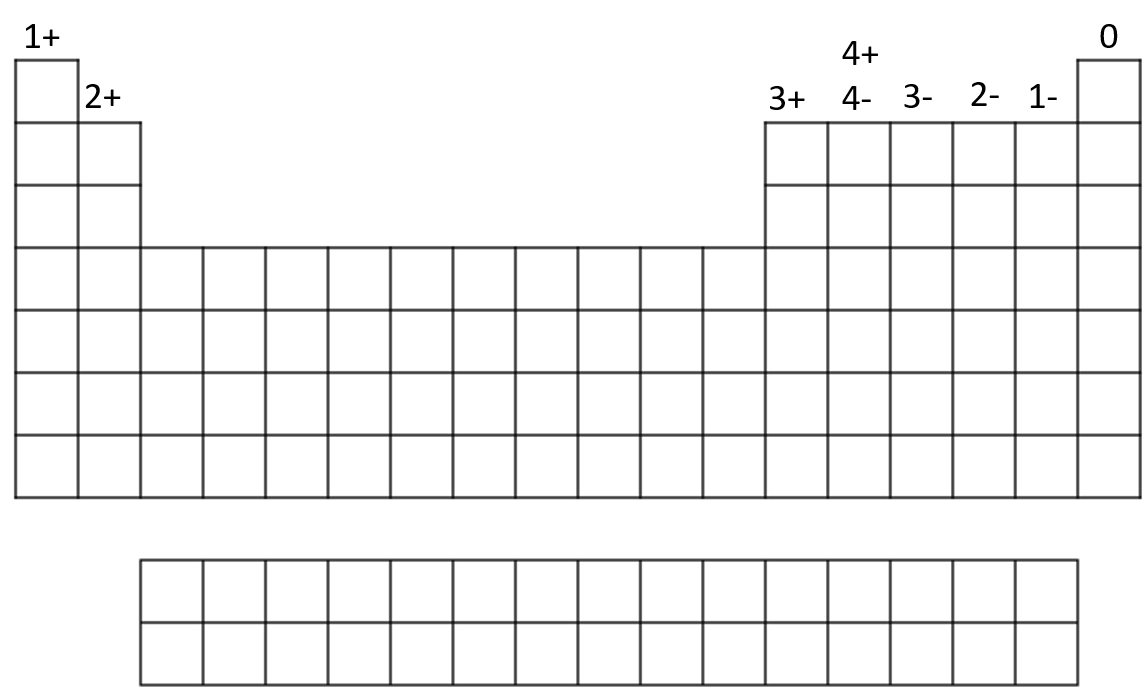
This table in figure 4 is a generalisation which works in most instances, but elements such as carbon and sulfur can have different oxidation numbers depending on the elements or ions they are bonding with.
Rules for assigning oxidation numbers
There are several rules that you need to know about oxidation numbers, and these are listed below:
- A molecule consisting of only one element always has an oxidation number of zero, since it is neutral. This applies even if the element is diatomic e.g. O2 or Cl2. These substances are called ‘free elements’.
For example, the oxidation number of hydrogen (H2) is [latex]\scriptsize \displaystyle 0[/latex]. The oxidation number of bromine (Br2) is also [latex]\scriptsize \displaystyle ~0[/latex]. - Monatomic ions (ions with only one element or type of atom) have an oxidation number that is equal to the charge on the ion.
For example, the chloride ion Cl– has an oxidation number of [latex]\scriptsize ^{-}1[/latex], and the magnesium ion Mg2+ has an oxidation number of [latex]\scriptsize ^{+}2[/latex]. - In a molecule or compound, the sum of the oxidation numbers for each element in the molecule or compound will be zero.
For example, the sum of the oxidation numbers for the elements in water will be [latex]\scriptsize 0[/latex]. - In a polyatomic ion (an ion made of different elements) the sum of the oxidation numbers is equal to the charge.
For example, the sum of the oxidation numbers for the elements in the sulfate ion ([latex]\scriptsize \text{SO}_{4}^{{2-}}[/latex]) will be [latex]\scriptsize {}^{-}2[/latex]. - An oxygen atom in a compound usually has an oxidation number of [latex]\scriptsize {}^{-}2[/latex] . One exception is in peroxides (e.g. hydrogen peroxide) when oxygen has an oxidation number of [latex]\scriptsize ^{-}1[/latex].
For example oxygen in water will have an oxidation number of [latex]\scriptsize {}^{-}2[/latex] while in hydrogen peroxide (H2O2) it will have an oxidation number of [latex]\scriptsize ^{-}1[/latex]. - The oxidation number of hydrogen is often [latex]\scriptsize ^{+}1[/latex]. One exception is in the metal hydrides where the oxidation number is [latex]\scriptsize ^{-}1[/latex].
For example, the oxidation number of the hydrogen atom in water is [latex]\scriptsize ^{+}1[/latex], while the oxidation number of hydrogen in lithium hydride (LiH) is [latex]\scriptsize ^{-}1[/latex]. - The oxidation number for Fluorine is [latex]\scriptsize ^{-}1[/latex].
You will notice that some elements always have the same oxidation number while other elements can change oxidation numbers depending on the compound they are in.
Summary of rules for specific atoms or periodic table groups
The abbreviation stands for oxidation number
A. For group 1: O.N. = [latex]\scriptsize \displaystyle ^{+}1[/latex] in all compounds
B. For group 2: O.N. = [latex]\scriptsize \displaystyle ^{+}2[/latex] in all compounds
C. For hydrogen: O.N. = [latex]\scriptsize \displaystyle ^{+}1[/latex] in combination with non-metals
O.N. = [latex]\scriptsize \displaystyle ^{-}1[/latex] in combination with metals and boron
D. For fluorine: O.N. = [latex]\scriptsize \displaystyle ^{-}1[/latex] in all compounds
E. For oxygen: O.N. = [latex]\scriptsize \displaystyle ^{-}1[/latex] in peroxides
O.N. = [latex]\scriptsize \displaystyle ^{-}2[/latex] in all other compounds (except with F)
F. For group 17: O.N. = [latex]\scriptsize \displaystyle ^{-}1[/latex] in combination with metals, non-metals (except O), and other halogens lower in the group
Let’s now look at an example.
Example 3.1
What is the oxidation number of sulfur in a sulfate ion?
Solution
Step 1: Determine the oxidation number for each atom
Oxygen will have an oxidation number of [latex]\scriptsize {}^{-}2[/latex] (rule 5, this is not a peroxide). The oxidation number of sulfur at this stage is uncertain since sulfur does not have a set oxidation number.
Step 2: Determine the oxidation number of sulfur by using the fact that the oxidation numbers of the atoms must add up to the charge on the compound
In the polyatomic ion [latex]\scriptsize \text{SO}_{4}^{{2-}}[/latex], the sum of the oxidation numbers must be [latex]\scriptsize {}^{-}2[/latex] (rule 4).
Let the oxidation number of sulfur be x. We know that oxygen has an oxidation number of [latex]\scriptsize {}^{-}2[/latex] and since there are four oxygen atoms in the sulfate ion, then the sum of the oxidation numbers of these four oxygen atoms is [latex]\scriptsize ^{-}8[/latex].
Step 3: Put this information together
[latex]\scriptsize x+{{(}^{-}}8)x{{=}^{-}}2+8{{=}^{+}}6[/latex]
So, the O.N. of sulfur is [latex]\scriptsize ^{+}6[/latex] in a sulfate ion.
Example 3.2
Give the oxidation number of both elements in ammonia (NH3).
Solution
Step 1: Determine the oxidation number for each atom
Hydrogen will have an oxidation number of [latex]\scriptsize {}^{+}1[/latex] (rule 6, ammonia is not a metal hydride). At this stage we do not know the oxidation number for nitrogen.
Step 2: Determine the oxidation number of nitrogen
Use the fact that the oxidation numbers of the atoms must add up to the charge on the compound.
In the compound NH3, the sum of the oxidation numbers must be [latex]\scriptsize 0[/latex] (rule 3).
Let the oxidation number of nitrogen be x. We know that hydrogen has an oxidation number of [latex]\scriptsize ^{+}1[/latex] and since there are three hydrogen atoms in the ammonia molecule, the sum of the oxidation numbers of these three hydrogen atoms is [latex]\scriptsize ^{+}3[/latex].
Step 3: Put this information together
[latex]\scriptsize x+{{(}^{+}}3)=0{{=}^{-}}3[/latex]
So, the O.N. of nitrogen is [latex]\scriptsize {}^{-}3[/latex].
Hydrogen has an O.N. of [latex]\scriptsize {}^{+}1[/latex] and nitrogen has an O.N. of [latex]\scriptsize {}^{-}3[/latex] in NH3.
Example 3.3
Give the oxidation numbers for all the atoms in sodium chloride (NaCl).
Solution
This is an ionic compound composed of Na+ and Cl– ions. Using rule 2, or the periodic table in figure 4, the oxidation number for the sodium ion is [latex]\scriptsize {}^{+}1[/latex] and for the chlorine ion it is [latex]\scriptsize {}^{-}1[/latex]. This then gives us a sum of [latex]\scriptsize 0[/latex] for the compound.
The O.N. for sodium is [latex]\scriptsize {}^{+}1[/latex] and for chlorine it is [latex]\scriptsize {}^{-}1[/latex] in NaCl.
Exercise 3.2
- Which of the following statements is true?
- Hydrogen can only have one possible oxidation number.
- The oxidation number of chlorine is always [latex]\scriptsize {}^{+}1[/latex].
- The oxidation number of oxygen is always [latex]\scriptsize {}^{-}2[/latex].
- The oxidation number of fluorine is always [latex]\scriptsize {}^{-}1[/latex].
- What is the oxidation number of Ba in BaCl2?
- [latex]\scriptsize {}^{+}2[/latex]
- [latex]\scriptsize {}^{+}1[/latex]
- [latex]\scriptsize {}^{-}2[/latex]
- [latex]\scriptsize {}^{-}1[/latex]
- When assigning oxidation numbers to elements in a compound, the sum of all the charges must equal:
- a positive value.
- zero.
- a negative value.
- Give the oxidation number for each element in the following compounds:
- MgF2
- KCl
- CO2
The full solutions can be found at the end of the unit.
Note
For a further explanation on oxidation numbers you can watch these two videos:
Oxidation numbers and oxidation and reduction
By looking at how the oxidation number of an element changes during a reaction, we can easily see whether that element is being oxidised (losing electrons) or reduced (gaining electrons).
If the oxidation number of a species becomes more positive, the species has been oxidised and if the oxidation number of a species becomes more negative, the species has been reduced.
Example 3.4
Explain the reaction between magnesium and chlorine to form magnesium chloride.
Solution
The chemical equation for this reaction is: [latex]\scriptsize \text{Mg+C}{{\text{l}}_{\text{2}}}\to \text{MgC}{{\text{l}}_{\text{2}}}[/latex].
As a reactant, magnesium has an oxidation number of zero, but as part of the product magnesium chloride, the element has an oxidation number of [latex]\scriptsize {}^{+}2[/latex]. This indicates that magnesium has lost two electrons in the reaction and has therefore been oxidised (note how the oxidation number becomes more positive). This can be written as a half-reaction. The half-reaction for this change is: [latex]\scriptsize \text{Mg}\to \text{M}{{\text{g}}^{{2+}}}+2{{e}^{-}}[/latex].
As a reactant, chlorine has an oxidation number of zero, but as part of the product magnesium chloride, the element has an oxidation number of [latex]\scriptsize ^{-}1[/latex]. This indicates that each chlorine atom has gained an electron and the element has therefore been reduced (note how the oxidation number becomes more negative). The half-reaction for this change is: [latex]\scriptsize \text{C}{{\text{l}}_{\text{2}}}+2{{e}^{-}}\to 2\text{C}{{\text{l}}^{-}}[/latex]
In the two half-reactions for a redox reaction the number of electrons donated is exactly the same as the number of electrons accepted. We will use this to help us balance redox reactions.
Summary
In this unit you have learnt the following:
- An oxidation number is the charge an atom would have if it was in a compound composed of ions and is used to determine whether a substance has gained or lost electrons during a redox reaction.
Unit 3: Assessment
Suggested time to complete: 30 minutes
- The oxidation number of any free element is:
- positive.
- zero.
- negative.
- The oxidation number of any alkali metal ion is:
- [latex]\scriptsize {}^{-}2[/latex]
- [latex]\scriptsize 0[/latex]
- [latex]\scriptsize {}^{+}1[/latex]
- When assigning oxidation numbers to elements in covalent compounds, the __________ will have the negative oxidation number.
- more metallic element
- diatomic element
- more electronegative element
- The goal of a redox reaction is to keep the number of electrons lost:
- less than the number of electrons gained.
- equal to the number of electrons gained.
- more than the number of electrons gained.
- Consider the following chemical equations:
[latex]\scriptsize \text{Fe}\to \text{F}{{\text{e}}_{2}}+2{{e}^{-}}[/latex]
[latex]\scriptsize \text{4}{{\text{H}}^{+}}\text{+}{{\text{O}}_{2}}+4{{e}^{-}}\to 2{{\text{H}}_{\text{2}}}\text{O}[/latex]
.
Which one of the following statements is correct?- Fe is oxidised and H+ is reduced.
- Fe is reduced and O2 is oxidised.
- Fe is oxidised and O2 is reduced.
- Fe is reduced and H+ is oxidised.
- What is the oxidation number of the oxygen atom in each of the following compounds?
- H2O2
- H2O
- Give the oxidation numbers for each of the elements in the reaction to produce the compound carbon dioxide. State if there is any difference between the oxidation number of the element in the reactant and the element in the product and what this indicates about the transfer of electrons.
[latex]\scriptsize \text{C+}{{\text{O}}_{2}}\to \text{C}{{\text{O}}_{2}}[/latex]
The full solutions can be found at the end of the unit.
Unit 3: Solutions
Exercise 3.1
- d. Loss of electrons. Oxidation – oxidation means the loss of electrons and can change an element to a positively charged ion
- Oxidation and reduction occur: a. simultaneously – Redox half reactions happen at the same time
- The species that is being reduced will have: a. a more negative oxidation state after the reduction has occurred. Reduction is the gaining of electrons – electrons are negatively charged.
- Simply stated, the gain of electrons is: a. reduction
- b. The species which is losing electrons. The reducing agent is the species which is being oxidised
Exercise 3.2
- d – see rule 7
- a – see rule 2
- b – see rule 3
- .
- In the compound MgF2, the oxidation number of fluorine is [latex]\scriptsize ^{-}1[/latex] (rule 7).
Let the oxidation number of magnesium be x. We know that fluorine has an oxidation number of [latex]\scriptsize ^{-}1[/latex] and since there are two fluorine atoms in the compound, the sum of the oxidation numbers of these two fluorine atoms is [latex]\scriptsize {}^{-}2[/latex].
[latex]\scriptsize x+({}^{-}2)=0={}^{+}2[/latex]
So, the oxidation number of magnesium is [latex]\scriptsize {}^{+}2[/latex].
Magnesium has an oxidation number of [latex]\scriptsize {}^{+}2[/latex] and fluorine has an oxidation number of [latex]\scriptsize ^{-}1[/latex]. - KCl is an ionic compound. Using rule 2, the oxidation number for K is [latex]\scriptsize {}^{+}1[/latex] and for Cl it is [latex]\scriptsize ^{-}1[/latex].
[latex]\scriptsize {}^{+}1+{}^{-}1=0[/latex]
So, the O.N. for K is [latex]\scriptsize {}^{+}1[/latex] and for Cl it is [latex]\scriptsize ^{-}1[/latex] - In the compound CO2, the sum of the oxidation numbers must be [latex]\scriptsize \displaystyle 0[/latex] (rule 3).
Let the oxidation number of carbon be x. We know that oxygen has an oxidation number of [latex]\scriptsize {}^{-}2[/latex] and since there are two oxygen atoms in the molecule, then the sum of the oxidation numbers of these two oxygen atoms is [latex]\scriptsize {}^{-}4[/latex].
[latex]\scriptsize x+({}^{-}4)=0={}^{+}4[/latex]
The O.N. of carbon is [latex]\scriptsize {}^{+}4[/latex] and oxygen is [latex]\scriptsize {}^{-}2[/latex] .
- In the compound MgF2, the oxidation number of fluorine is [latex]\scriptsize ^{-}1[/latex] (rule 7).
Unit 3: Assessment
- b. zero
- c. [latex]\scriptsize {}^{+}1[/latex]
- c. more electronegative element
- b. equal to the number of electrons gained
- c. Fe is oxidised and O2 is reduced
- .
- Hydrogen = [latex]\scriptsize ^{+}1[/latex]. 2 x H =[latex]\scriptsize {}^{+}2[/latex]. Oxygen = [latex]\scriptsize x[/latex]
[latex]\scriptsize 2x+({}^{+}2)x=0={}^{-}1[/latex]
O.N. of oxygen is [latex]\scriptsize {}^{-}1[/latex] - This compound is also not a peroxide, so the oxidation number of oxygen is [latex]\scriptsize {}^{-}2[/latex] (rule 5).
- Hydrogen = [latex]\scriptsize ^{+}1[/latex]. 2 x H =[latex]\scriptsize {}^{+}2[/latex]. Oxygen = [latex]\scriptsize x[/latex]
- O.N. of C = [latex]\scriptsize x[/latex]
O.N. of O2 = [latex]\scriptsize ^{-}4[/latex]
[latex]\scriptsize x+({}^{-}4)=0={}^{+}4[/latex]
O.N. of C = [latex]\scriptsize {}^{+}4[/latex]
The oxidation number of carbon in the products is [latex]\scriptsize 0[/latex] and in the reactants it is [latex]\scriptsize {}^{+}4[/latex] The oxidation number has increased (become more positive). Carbon has lost electrons in an oxidation half reaction.
.
The oxidation number of oxygen in the products is [latex]\scriptsize 0[/latex] and in the reactants it is [latex]\scriptsize {}^{-}2[/latex]. The oxidation number has decreased (become more negative). Oxygen has gained electrons in a reduction half reaction.
Media Attributions
- Fig 1 © DHET is licensed under a CC BY (Attribution) license
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
- Fig 3 © Zbysiu Rodak is licensed under a CC0 (Creative Commons Zero) license
- Fig 4 © DHET is licensed under a CC BY (Attribution) license
the loss of electrons
the gain of electrons
the elements which are being oxidised
the elements which are being reduced
involve the transfer of electrons from one substance to another
the word used in chemistry to indicate either a compound, a molecule, an ion, an atom, or an element
either the oxidation or reduction reaction parts of a redox reaction
the charge an atom would have if it were in a compound composed of ions
oxidation number
