Mechanical Properties of matter: Describe, analyse and apply principles heat and thermal properties of solids and liquid
Unit 1: Expansion of solids and liquids
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Describe and apply expansion of solids (refer to linear, area and volume expansion).
- Describe and apply expansion of liquids and viscosity.
What you should know
Before you start this unit, make sure you can:
- Understand kinetic energy and its relationship to the temperature and movement of particles. Refer to level 2 subject outcome 5.1 unit 1 to revise this.
Introduction
In this unit we learn about thermal expansion. The expansion of alcohol in a thermometer is one of many commonly encountered examples of . Thermal expansion is the change in size or volume of a given mass with temperature. Hot air rises because its volume increases, which causes the hot air’s density to be smaller than the density of surrounding air, causing a buoyant (upward) force on the hot air. The same happens in all liquids and gases, driving natural heat transfer upwards in homes, oceans, and weather systems. Solids also undergo thermal expansion. Railroad tracks and bridges, for example, have expansion joints to allow them to freely expand and contract with temperature changes.

Bridges made of steel girders also expand during the day and contract during the night. They will bend if their ends are fixed. To allow for this a thermal girder rests on rollers in the gap left for expansion.
Thermal expansion
It is a well-known phenomenon that substances expand on heating and contract on cooling. If you heat a body, it alters its dimensions, depending on the shape of the body.
Thermal expansion is related to temperature change. The greater the temperature change, the more a strip will bend. Secondly, it depends on the material. In a thermometer, for example, the expansion of alcohol is much greater than the expansion of the glass containing it.
As is discussed in level 2 subject outcome 5.1, an increase in temperature means an increase in the average kinetic energy of the individual atoms. In a solid, unlike in a gas, the atoms or molecules are closely packed together, but their kinetic energy (in the form of small, rapid vibrations) pushes neighbouring atoms or molecules apart from each other. This neighbour-to-neighbour pushing results in a slightly greater distance, on average, between neighbours, and adds up to a larger size for the whole body. For most substances under ordinary conditions, there is no one direction, and an increase in temperature will increase the solid’s size by a certain fraction in all dimensions.
Thermal expansion can be defined as the change in the length, width, height, or volume of any material on changing the temperature. Thermal expansion is very evident in solids as atoms are densely packed. Thermal expansion of solids has many applications in day-to-day life.
Objects expand in all dimensions, as illustrated in figure 2 below. That is, their areas and volumes, as well as their lengths, increase with temperature. Holes also get larger with temperature. If you cut a hole in a metal plate, the remaining material will expand exactly as it would if the plug was still in place. The plug would get bigger, and so the hole must get bigger too. (Think of the ring of neighbouring atoms or molecules on the wall of the hole as pushing each other farther apart as temperature increases. Obviously, the ring of neighbours must get slightly larger, so the hole gets slightly larger).
There are three types of common expansion.
- The expansion that occurs in length is called .
- If we take a square tile and heat it, the expansion will be on two fronts, length, and breadth, and this is called .
- If we take a cube and heat it, all its sides expand and now the body experiences an increase in the overall volume due to this and this is called .
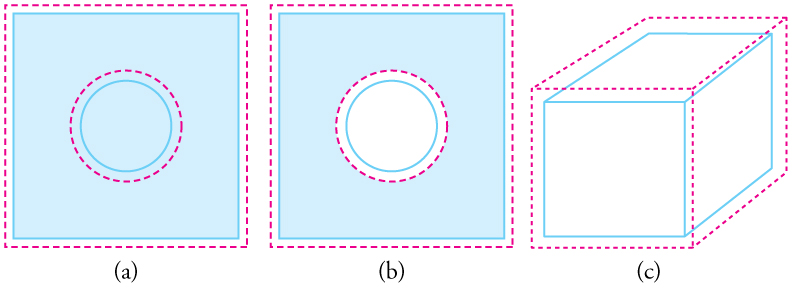
In figure 2, the drawing (a) area increases because both length and width increase. The area of a circular plug also increases. In drawing (b), if the plug is removed, the hole it leaves becomes larger with increasing temperature, just as if the expanding plug were still in place. In drawing (c) the volume also increases, because all three dimensions increase.
The coefficient of thermal expansion describes how the size of an object changes with a change in temperature. Specifically, it measures the fractional change in size per degree change in temperature at a constant pressure, such that lower coefficients describe lower tendency for change in size. The expansion of a solid when heated is small. A metal ruler may expand by when heated. Though small, this expansion can create a very large force if it is restrained.
The volumetric thermal expansion coefficient is the most basic thermal expansion coefficient, and the most relevant for fluids. In general, substances expand or contract when their temperature changes, with expansion or contraction occurring in all directions.
Liquid particles are usually less tightly attracted to neighbouring molecules, they generally move further than solid particles when heated. Hence, liquid expands more than a solid if the temperature rise is the same. This expansion of liquid may be used in a liquid-in-glass thermometer. The volume increase of alcohol or mercury may be calibrated to provide a temperature reading since the expansion is almost directly proportional to the temperature rise.
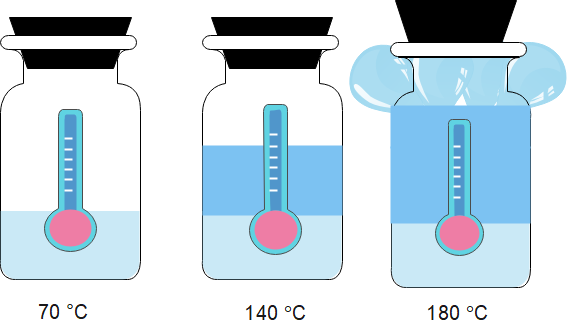
Bulb thermometers (glass thermometers) exploit the property of thermal expansion of materials to put a number on the amount of internal heat of a substance.
Figure 4 shows a thermometer at a relatively low temperature (on the left).
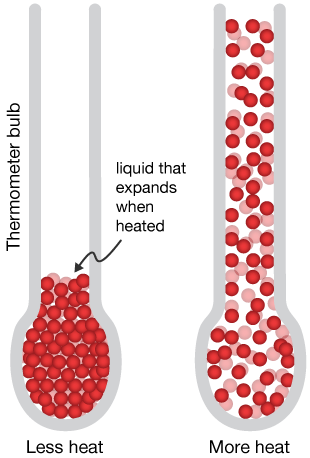
When the bulb is exposed to a material with more heat energy, the atoms/molecules of the material collide with the glass of the bulb, which transfers the collision energy to the molecules of the thermometer liquid, which absorb that energy and so the volume of the liquid expands. It is not the particles which expand, but the space between the particles that increases.
A number of materials contract on heating within certain temperature ranges; this is usually called negative thermal expansion, rather than ‘thermal contraction’. For example, the coefficient of thermal expansion of water drops to zero as it is cooled to [latex]\scriptsize \displaystyle 3.983~^\circ\text{C}[/latex] and then becomes negative below this temperature; this means that water has a maximum density at this temperature, and this leads to bodies of water maintaining this temperature at their lower depths during extended periods of sub-zero weather.
Factors affecting thermal expansion
Unlike gases or liquids, solid materials tend to keep their shape when undergoing thermal expansion. Thermal expansion generally decreases with increasing bond energy, which also influences the melting point of solids, so, high melting point materials are more likely to have lower thermal expansion.
In general, liquids expand slightly more than solids. The thermal expansion of glass is higher compared to that of crystals.
Absorption or desorption of water (or other solvents) can change the size of many common materials; many materials change size much more due to this effect than due to thermal expansion. Common plastics exposed to water can, in the long term, expand by many percent.
Thermal expansion changes the space between particles of a substance, which changes the volume of the substance so changing its density. This plays a crucial role in convection of unevenly heated fluid masses, such as making thermal expansion partly responsible for wind and ocean currents.
In general, objects will expand with increasing temperature. Water is the most important exception to this rule. Water expands with increasing temperature (its density decreases) when it is at temperatures greater than [latex]\scriptsize \displaystyle 4\text{ }^\circ \text{C}[/latex]. However, it expands with decreasing temperature when it is between [latex]\scriptsize \displaystyle 4\text{ }^\circ \text{C}[/latex] and [latex]\scriptsize 0\text{ }^\circ \text{C}[/latex]. Water is densest at [latex]\scriptsize \displaystyle 4\text{ }^\circ \text{C}[/latex].
Perhaps the most striking effect of this phenomenon is the freezing of water in a pond. When water near the surface cools down to [latex]\scriptsize \displaystyle 4\text{ }^\circ \text{C}[/latex] it is denser than the remaining water and so it will sink to the bottom. This ‘turnover’ results in a layer of warmer water near the surface, which is then cooled. Eventually the pond has a uniform temperature of [latex]\scriptsize \displaystyle 4\text{ }^\circ \text{C}[/latex]. If the temperature in the surface layer drops below [latex]\scriptsize \displaystyle 4\text{ }^\circ \text{C}[/latex], the water is less dense than the water below, and so it stays near the top. As a result, the pond surface can completely freeze over. The ice on top of liquid water provides an insulating layer from winter’s harsh exterior air temperatures. Fish and other aquatic life can survive in [latex]\scriptsize \displaystyle 4\text{ }^\circ \text{C}[/latex] water beneath ice, due to this unusual characteristic of water. It also produces circulation of water in the pond that is necessary for a healthy ecosystem of the body of water.
Applications of thermal expansion in everyday life
Thermal expansion is used in many applications in our daily life.
- Thermometers: In thermometers, thermal expansion is used in temperature measurements.
- Removing tight lids: To open the cap of a bottle that is tight enough, immerse it in hot water for a minute or so. Metal caps expand and become loose and therefore easier to turn and open.
- Riveting: To join steel plates tightly together, red hot rivets are forced through holes in the plates. The end of hot rivets is then hammered. On cooling, the rivets contract and bring the plates tightly together.
- Fixing metal tyres on wooden wheels: Iron rims are fixed on wooden wheels of carts. Iron rims are heated. The thermal expansion allows them to slip over the wooden wheel. Water is poured on them to cool. The rim contracts and becomes tight over the wheel.
- Railway tracks expand during a hot day. If the tracks are not designed for the expansion, the entire track may bend out of shape during expansion. Rails in direct sunshine can be as much as [latex]\scriptsize \displaystyle \text{20 }\!\!^\circ\!\!\text{ C}[/latex] hotter than air temperature. Because rails are made from steel, they expand as they get hotter, and can start to curve, known as ‘buckling’.

Railway tracks are lengths of rails joined by rail joints (joint bars, fishplates). If you look closely at the rails, you will find that every [latex]\scriptsize \displaystyle 12[/latex] or [latex]\scriptsize \displaystyle 24[/latex] metres, there will be a small gap between the two rails.
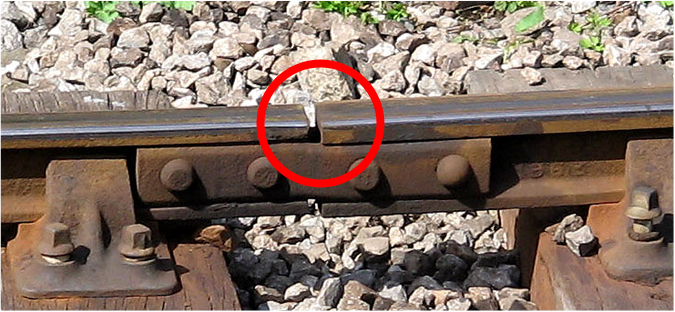
This is done to solve the problem of thermal expansion and contraction of railroad rails. If there is no such gap, the steel rails will squeeze, twist, and buckle when they are extremely heated, and deform the entire railway. When the weather is hot in summer, the length of the rails increases and the rails without reserved gaps can only bulge upwards, which is not good for safety. To avoid this phenomenon, a gap must be reserved between the rails.
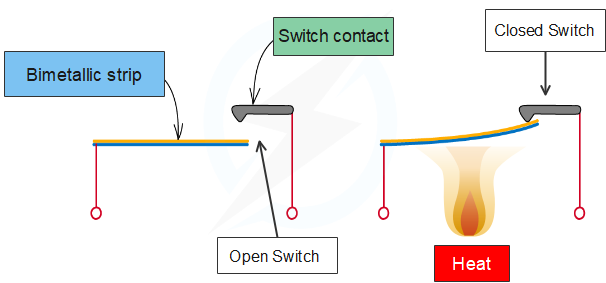
The expansion of solids can be put to good use. Two pieces of different metals with different expansion coefficients may be bound together. When the temperature changes, the two metals expand differently. This causes the strip to bend according to the temperature. This bimetallic strip may be used to open and close an electric circuit to control temperature.
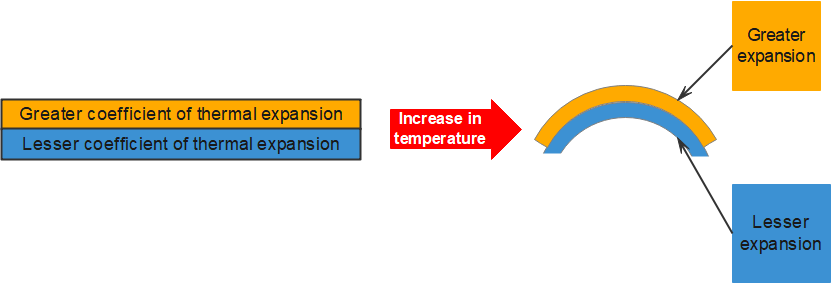
A bimetallic strip can be made to bend at a given temperature, forming a temperature-activated switch. A bimetallic strip consists of two thin strips of different metals such as brass and iron joined together (see figure 7). On heating the strip, brass expands more than iron. This unequal expansion causes the bending of the strip.
Bimetal strips are used for various purposes. Bimetal thermometers are used to measure temperature, especially in furnaces and ovens. Bimetal strips are used in thermostats. A bimetal thermostat is used to control the temperature of the heater coil in an electric iron.
One example of where a bimetallic strip is used is in petrol stations for the running of petrol from a freshly filled tank on a hot day. Gasoline starts out at the temperature of the ground under the gas station, which is cooler than the air temperature above. The gasoline cools the steel tank when it is filled. Both the gasoline and steel tank expand as they warm to air temperature, but gasoline expands much more than steel, and so it may overflow.
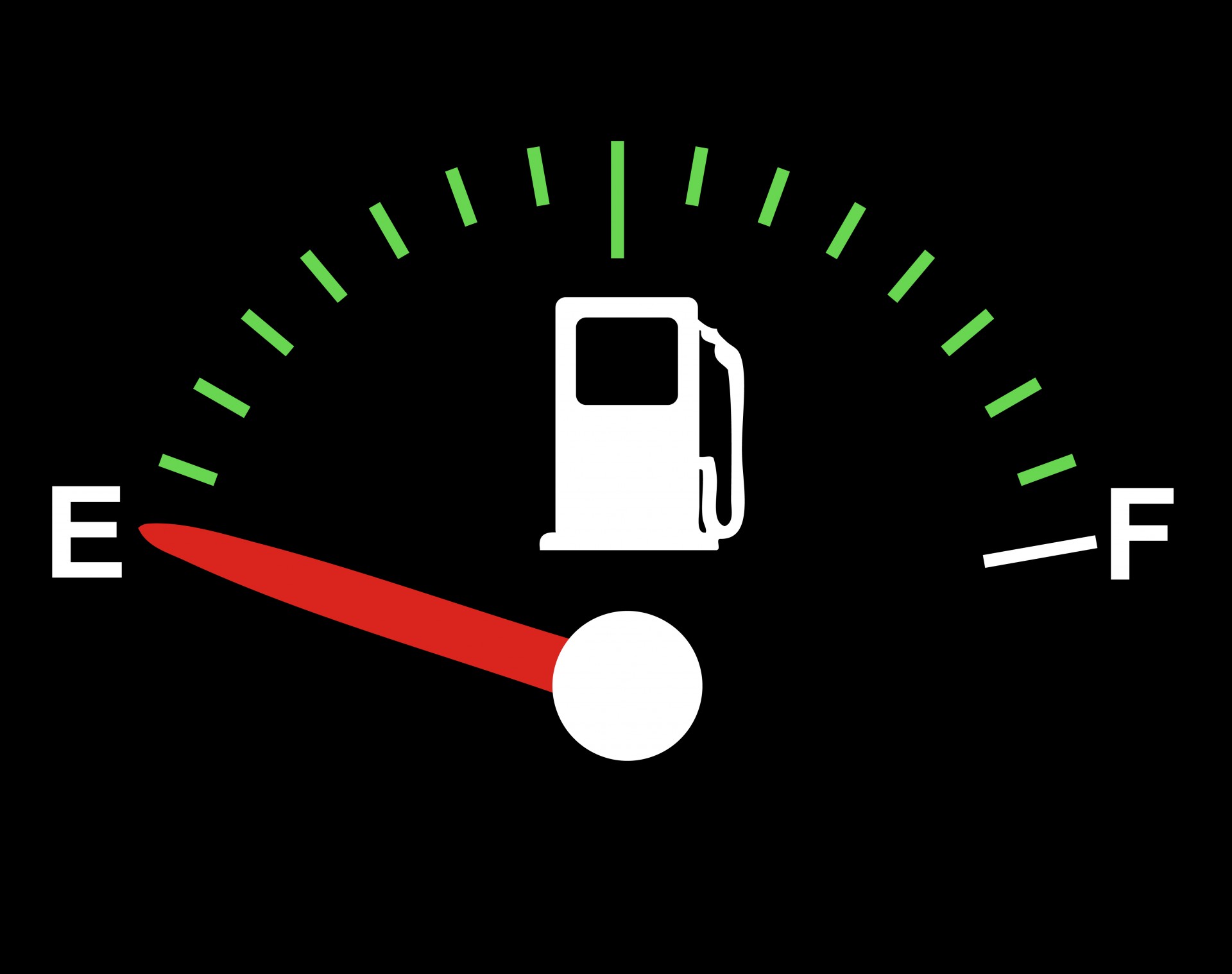
This difference in expansion can also cause problems when interpreting the petrol gauge in a car. The actual amount (mass) of petrol left in the tank when the gauge hits ‘empty’ is a lot less in the summer than in the winter. The petrol has the same volume as it does in the winter when the ‘petrol light goes on, but because the petrol has expanded, there is less mass. If you are used to getting another [latex]\scriptsize \displaystyle 40[/latex] kilometres on ‘empty’ tank in the winter, beware – you will probably run out much more quickly in summer.
Other examples of thermal expansion in our daily life include:
- cracks in the road when the road expands on heating
- sags in electrical power lines
- windows with metal frames need rubber spacers to avoid thermal expansion creating gaps
- tyres bursting on hot days when filled full of air.
is created by thermal expansion or contraction. Thermal stress can be destructive, such as when expanding petrol ruptures a tank. It can also be useful, for example, when two parts are joined together by heating one part in manufacturing, then slipping it over the other part and allowing the combination to cool. Thermal stress can explain many phenomena, such as the weathering of rocks and pavements by the expansion of ice when it freezes. Railroad tracks and roadways can buckle on hot days if they lack sufficient expansion joints (see figure 5.) Power lines sag more in the summer than in the winter and will snap in cold weather if there is insufficient slack. Cracks open and close in plaster walls as a house warms and cools. Glass cooking pans will crack if cooled rapidly or unevenly, because of differential contraction and the stresses it creates.
Note
To consolidate your understanding of thermal expansion, you can watch this video by JamJar called Thermal expansion of solids, liquids and gases.
Exercise 1.1
- List the three types of expansion an object or substance can experience.
- Liquids expand when they are heated. Using this knowledge, explain why you should fill your car with petrol or diesel in the early morning in summer.
- Explain why powerlines sag more in the summer.
The full solutions are at the end of the unit.
Viscosity of liquids
is a measure of how resistant a substance is to motion. Most fluids offer some resistance to motion, and we call this resistance ‘viscosity’. Viscosity arises when there is relative motion between layers of the fluid. More precisely, it measures resistance to flow arising due to the internal friction between the fluid layers as they slip past one another when fluid flows. Viscosity can also be thought of as a measure of a fluid’s thickness or its resistance to objects passing through it.
A fluid with large viscosity resists motion because its strong intermolecular forces give it a high internal friction, resisting the movement of layers past one another. On the contrary, a fluid with low viscosity flows easily because its molecular makeup results in very little friction when it is in motion. Gases also exhibit viscosity, but it is harder to notice in ordinary circumstances.
Take note!
Viscosity is a measurement of how resistant a fluid is when attempts are made to move through it.
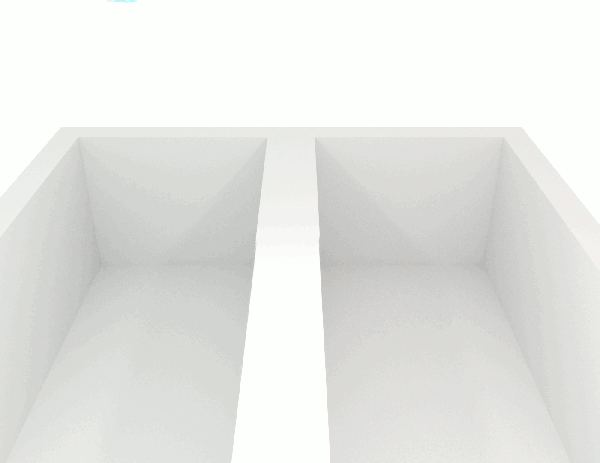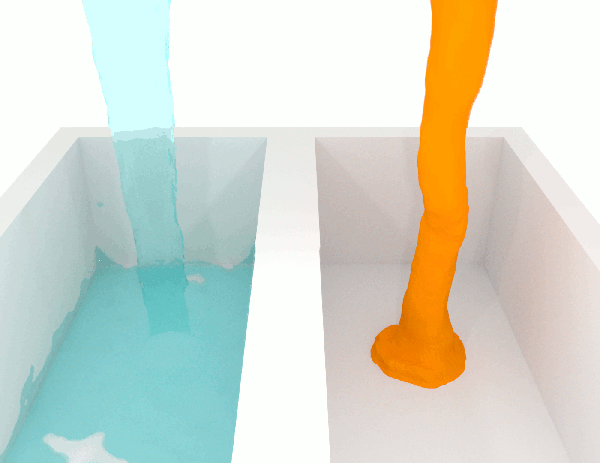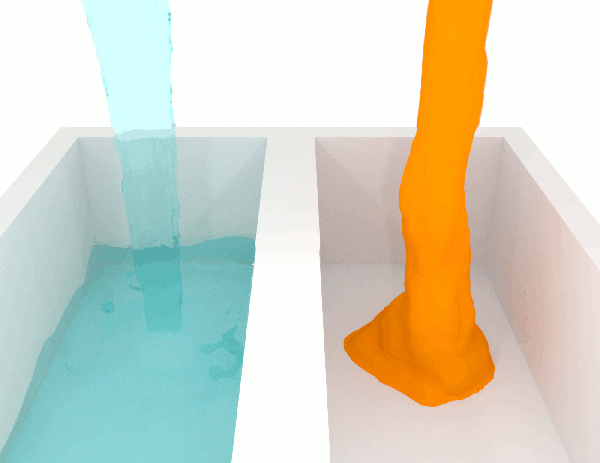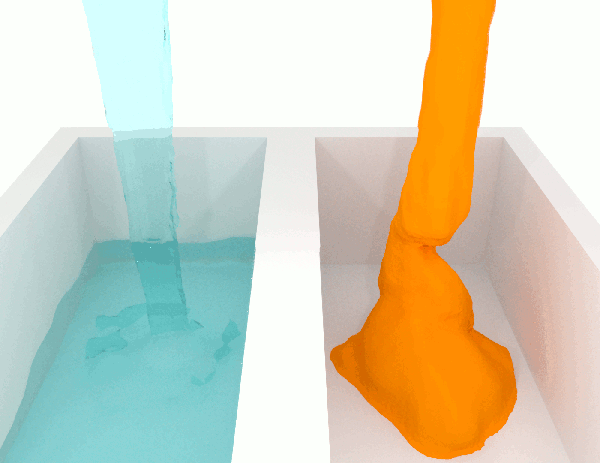
A fluid with low viscosity of said to be ‘thin’, whilst a high viscosity is said the be ‘thick’ and more viscous.
A low-viscosity substance will flow much more easily than a high-viscosity substance. For this reason, if you are looking for an adhesive that will stay put after it’s dispensed, for example, you will want one with high viscosity. Meanwhile, if you need one that will spread out easily, you’ll need one with low viscosity.
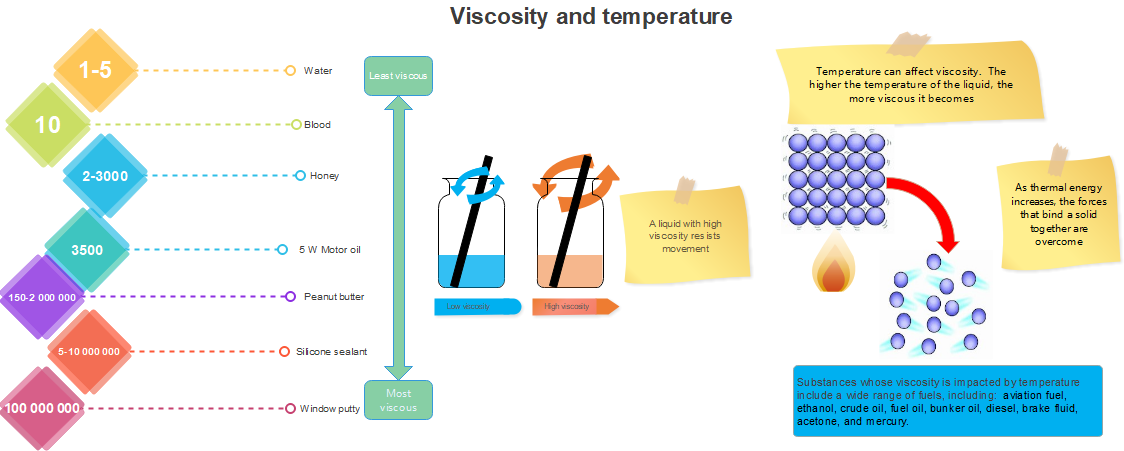
Figure 11: The viscosity of a substance is affected by its temperatureThe viscosity of liquids decreases rapidly with an increase in temperature, and the viscosity of gases increases with an increase in temperature. So, upon heating, liquids flow more easily, whereas gases flow more slowly.
With regard to machinery that uses oil for lubrication of moving parts. If the oil’s viscosity becomes too high, the oil’s flow will be restricted. As a result, the machinery’s components will not have the lubrication they need. This will lead to metal-on-metal damage.
If you have a gearbox that uses oil and the oil is too thick due to cold temperatures, the gear will have to push chunks of the lubricant away. The oil will therefore not reach the other housing components that require lubrication. Using substances that are too viscous can also pose a safety concern.
Newtonian and non-Newtonian fluids
If the viscosity does not change with pressure, we describe something as being a . And, if the viscosity does change as the temperature changes, we describe something as being a .
The fluid whose viscosity remains constant is known as the Newtonian fluid. These fluids are independent of the amount of shear stress applied to them with respect to time. The relationship between the viscosity and shear stress of these fluids is linear.
Examples of Newtonian Fluids are:
- water
- alcohol
- mineral oil
- petrol.
A fluid in which viscosity changes when shear stress is applied is known as a non-Newtonian fluid. These fluids are the opposite of Newtonian fluids.
Examples of non-Newtonian Fluids are:
- toothpaste
- tomato sauce
- cosmetics
- paint.
Note
You can watch a video on non-Newtonian fluids by Sciencemandotcom called Physics – Non-Newtonian Fluids.
Summary
In this unit you have learnt the following:
- Thermal expansion is the increase, or decrease, of the size (length, area, or volume) of a body due to a change in temperature.
- Thermal expansion is relatively small, but not negligible, for liquids and solids.
- Linear thermal expansion is the change in length with change in temperature.
- Solids change in area due to thermal expansion.
- Liquids change in volume due to thermal expansion
- Thermal stress is created when thermal expansion is controlled.
- Viscosity is a measure of how resistant a substance is to motion
Unit 1: Assessment
Suggested time to complete: 15 minutes
- One method of getting a tight fit, say of a metal peg in a hole in a metal block, is to manufacture the peg slightly larger than the hole. The peg is then inserted when at a different temperature than the block. Should the block be hotter or colder than the peg during insertion? Explain your answer.
- Does it really help to run hot water over a tight metal lid on a glass jar before trying to open it? Explain your answer.
- Due to the strange behaviour of water, for the same mass, the volume of ice is more than that of water. Is this true or false? Explain your answer.
- When water is cooled from [latex]\scriptsize \displaystyle 4\text{ }^\circ \text{C}[/latex] to [latex]\scriptsize 0\text{ }^\circ \text{C}[/latex] does it:
- contract
- expand
- first contract then expand
- first expand and then contract?
Explain your answer.
- What is viscosity? Write a definition.
- How are viscosity and flow rate related?
The full solutions are at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- linear, area and volume
- Petrol comes out of the storage tank at the temperature of the ground under the petrol station, which is cooler than the air temperature above. Cooler petrol has a higher density and mass and is less likely to evaporate. So you get more fuel!
- Metals expand on heating. During the summer, the powerlines will heat up causing them to expand, meaning they will sag more.
Unit 1: Assessment
- The block should be warmer than the peg. Solids expand when heated. When the peg is placed in the hole, and the block cools, the block will contract, making a tight fit with the peg.
- Yes. As you heat the lid, it will expand, so it will be easier to open the jar.
- True. Generally, the volume of substances increases on heating and decreases on cooling. But when water is heated from [latex]\scriptsize 0\text{ }^\circ \text{C}[/latex] to [latex]\scriptsize 4\text{ }^\circ \text{C}[/latex] , its volume decreases and increases only after [latex]\scriptsize 4\text{ }^\circ \text{C}[/latex].
- The correct option is B. Expansion of water occurs when water is cooled from [latex]\scriptsize 4\text{ }^\circ \text{C}[/latex] to [latex]\scriptsize 0\text{ }^\circ \text{C}[/latex] since density begins to decrease after attaining maximum value at [latex]\scriptsize 4\text{ }^\circ \text{C}[/latex].
- Viscosity is a measure of a fluid’s resistance to flow.
- The viscosity of liquids decreases rapidly with an increase in temperature, and the viscosity of gases increases with an increase in temperature.
Media Attributions
- Fig 1 © Libretext is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 2 © Libretext is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 3 © DHET is licensed under a CC BY (Attribution) license
- Fig 4 © xactly is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 5 © U.S. Department of Transportation. is licensed under a Public Domain license
- Fig 6 © DHET is licensed under a CC BY (Attribution) license
- Fig 7 © DHET is licensed under a CC BY (Attribution) license
- Fig 8 © DHET is licensed under a CC BY (Attribution) license
- Fig 9 © Public domain pictures is licensed under a Public Domain license
- Fig 10 © Synapticrelay is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 11 © DHET is licensed under a CC BY (Attribution) license
the change in size or volume of an object with change in temperature
composed of two different metals
the change in length caused by a change in temperature
the change in the area of an object caused by a change in temperature
the change in volume caused by a change in temperature
stress caused by thermal expansion or contraction
the state of being thick, sticky, and semi-fluid in consistency, due to internal friction
any fluid that shows a viscosity that remains constant regardless of any external stress that is placed upon it, such as mixing or a sudden application of force
a liquid that behaves like a solid and remains in a semi-solid or highly viscous state when a sudden force is applied
