Chemical Systems and Industry: Identify and critically evaluate the impact of scientific knowledge on the atmosphere and the quality of human, environmental and socio-economic development
Unit 2: Air pollution
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Identify gases which are emitted by industry and explain why these gases are hazardous to human health.
- Describe and define air pollutants and methods of prevention.
What you should know
Before you start this unit, make sure you can:
- Identify industrial gases. Refer to level 3 subject outcome 7.1 unit 1 to revise this.
Introduction
Hazardous gases are emitted by industry in the manufacture of goods, by power stations when fossil fuels are burnt, in emissions from vehicles and aeroplanes, and from burning biomass. These gases cause air pollution. Air pollution refers to the introduction of harmful substances into the atmosphere. Air pollution has adverse effects on humans, other living organisms, and the environment either as solid particles, liquid droplets, or gases. Air pollution emanates from various sources, which include natural and sources. Natural sources of air pollution include volcanoes, which produce sulphur, chlorine, and particulates. Wildfires result in the production of smoke, carbon dioxide and carbon monoxide. Other natural sources of air pollution include domestic animals such as cattle, which release methane, and pine trees, which release . Most forms of air pollution we know today are because of human activities and include fossil fuel burning (coal, oil, and natural gas in industrial processes), electricity generation, vehicle emissions, aircraft emissions, domestic fuel burning, and the use of household materials that contain organic pollutants, biomass burning and waste incineration.
South Africa’s Air Quality Act of 2004 put in place various measures for the prevention of pollution and national norms and standards for the regulation of air quality in the country. Further additions have been made since then to include legislation to reduce the emissions of greenhouse gases, and air quality standards for the emissions of seven pollutants including ozone, carbon dioxide and sulfur dioxide.
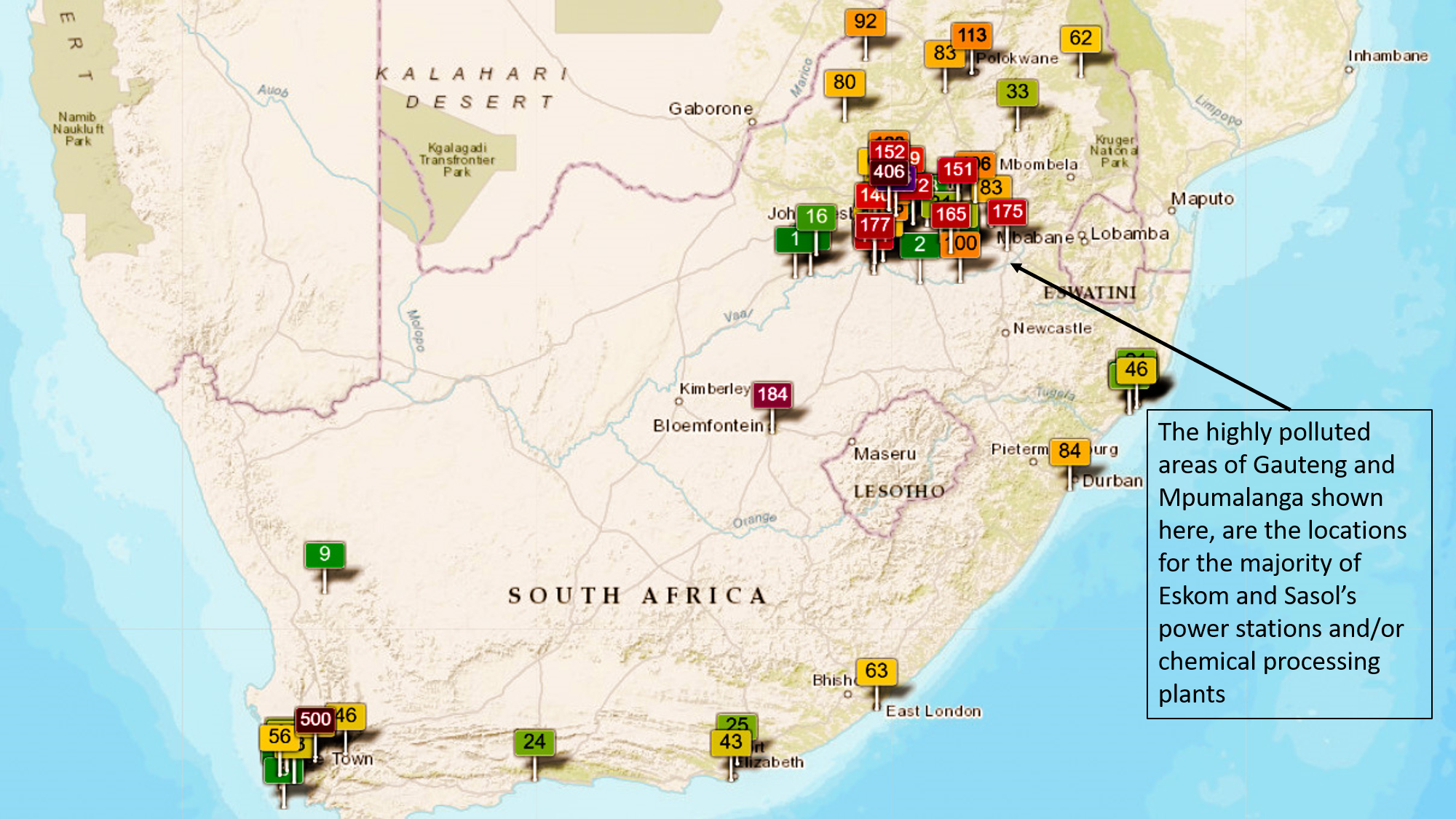
In 2020 the South African government stated that Eskom would not be allowed to operate its plants at current emission levels until their scheduled closure. The Minister for the Environemnt also advised Sasol that they would not be allowed to postpone installation of pollution abatement equipment at an oil refinery beyond March 2025. Together these two companies account for over half of the air polluting gases emitted in South Africa.
Hazardous gases released by Industry
Pollutants are categorised into two major types based on how they originated, namely primary and secondary pollutants. are those released directly from the source into the air in a harmful form. The primary pollutants that account for nearly all air pollution problems are carbon monoxide, VOCs, nitrogen oxides, sulfur dioxides and particulate material. are produced through reactions between primary pollutants and normal atmospheric compounds. For example, ground-level ozone forms over urban areas through reactions, powered by sunlight, between primary pollutants (oxides of nitrogen) and other atmospheric gases such as VOCs.
Primary pollutants
Below is a list of the most prevalent primary pollutants.
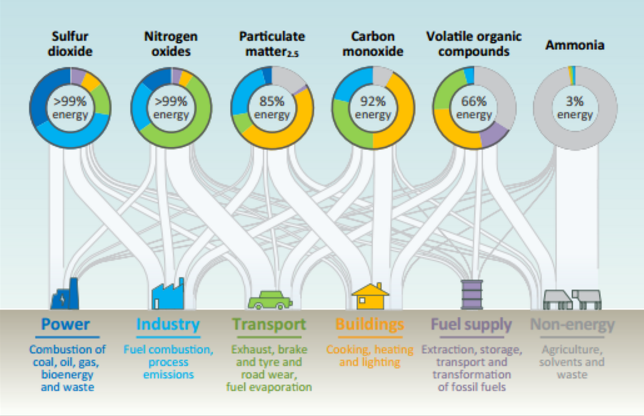
Carbon monoxide (CO) is a colourless, odourless gas emitted from combustion processes, specifically the incomplete combustion of fuel. Nationally and, particularly in urban areas, most of the CO emissions into the air come from moving sources such as vehicles. CO can cause harmful health effects by reducing oxygen delivery to the body’s organs (like the heart and brain) and tissues. At extremely high levels, CO can cause death.
Nitrogen dioxide (NO2) is one of a group of highly reactive gases known as ‘oxides of nitrogen’, or ‘nitrogen oxides (NOx)’. Other nitrogen oxides include nitrous acid and nitric acid. NO2 is a yellowish-brown to reddish-brown foul-smelling gas that is a major contributor to and . Nitrogen oxides result when atmospheric nitrogen and oxygen react at the high temperatures created by combustion engines. Most emissions come from combustion in vehicle engines, electrical utilities and industrial combustion.
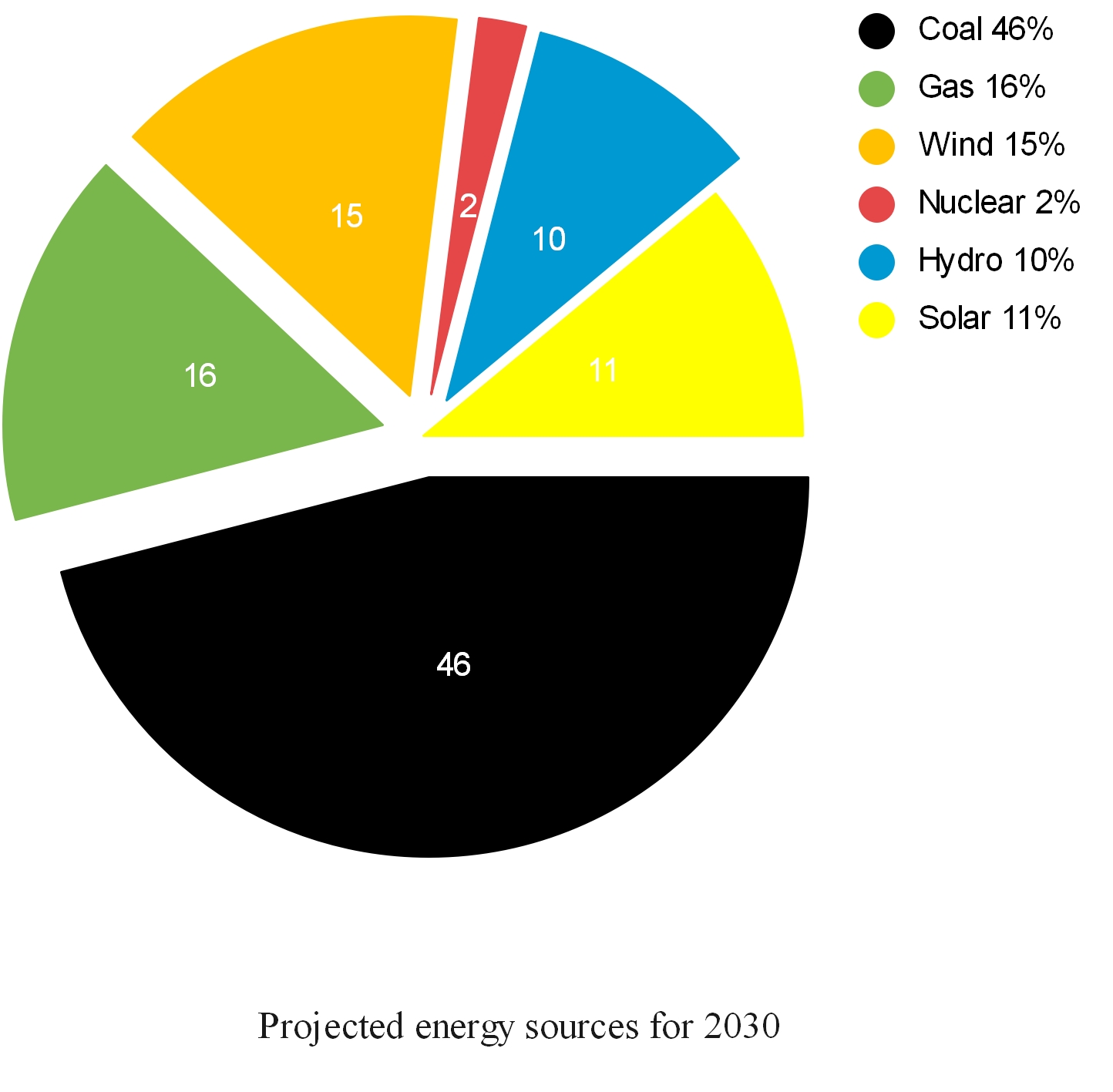
Sulfur dioxide (SO2) is one of a group of highly reactive gases known as ‘oxides of sulfur’. The largest sources of SO2 emissions are from fossil fuel combustion at power plants. Smaller sources of SO2 emissions include industrial processes such as extracting metals from their ores, and the burning of high sulfur containing fuels by trains, large ships, and non-road equipment.
Lead (Pb) is a metal found naturally in the environment as well as in manufactured products. The major sources of lead emissions have historically been from fuels in motor vehicles (such as cars and trucks) and industrial sources. As a result of the removal of lead from petrol, emissions of lead from the transportation sector dramatically declined by 95% between 1980 and 1999, and levels of lead in the air decreased by 94% during that time. The major sources of lead emissions today are ore and metal processing and piston-engine aircraft operating on leaded aviation fuel. Today, the highest levels of lead in air are usually found near lead smelters.
Volatile Organic Compounds (VOCs) are carbon-containing chemicals emitted as gases from natural and human-made sources. Natural sources of VOCs include plants, the largest source, and bacteria in the guts of termites and ruminant animals. These compounds are generally oxidised to carbon monoxide and carbon dioxide in the atmosphere. VOCs are of great concern because they are precursors for the formation of ozone, a secondary air pollutant.
Many synthetic organic chemicals such as benzene, toluene, formaldehyde, vinyl chloride, chloroform, and phenols are widely used as ingredients in countless household products. Paints, paint strippers, varnishes, many cleaning, disinfecting, cosmetic, and degreasing materials contain VOCs. Fuels are also made up of organic chemicals. All these products can release organic compounds while you are using them, and, to some degree, when they are stored. The ‘new car smell’ characteristic of new cars is from a complex mix of dozens of VOCs. Also, concentrations of many VOCs are consistently higher indoors (up to ten times higher) than outdoors.
Particulate material (PM), sometimes known simply as ‘particulates’ refers to solid particles and liquid droplets suspended in the air we breathe. Particulate pollution is made up of a variety of components, including acids (nitrates and sulfates), organic chemicals, metals, soil or dust particles, and allergens (pollen and mould spores). The size of the particles are linked to their potential for causing health problems. Particles that are [latex]\scriptsize \displaystyle 10[/latex] micrometres in diameter or smaller generally pass through the throat and nose and enter the lungs.
Particulate material is grouped into two types: ‘inhalable coarse particles’, with diameters larger than [latex]\scriptsize \displaystyle 2.5[/latex] micrometres and smaller than [latex]\scriptsize \displaystyle 10[/latex] micrometres and ‘fine particles’, with diameters that are [latex]\scriptsize \displaystyle 2.5[/latex] micrometres and smaller. Our respiratory systems are equipped to filter larger particles out of the air once it is inhaled.
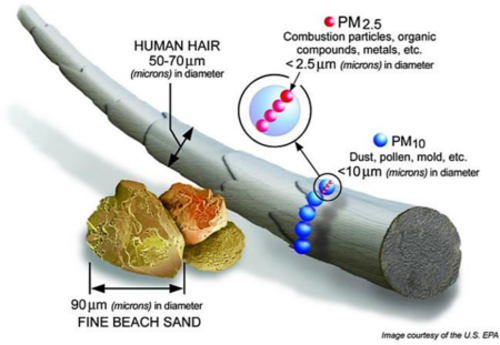
The lungs are vulnerable to both coarse particles (PM [latex]\scriptsize \displaystyle 10[/latex]), and fine particles (PM [latex]\scriptsize \displaystyle 2.5[/latex]). These can slip past the respiratory system’s natural defences and get deep into the lungs and some may even get into the bloodstream. Coarse particles come from road dust while fine particles come from combustion processes.
Secondary sources
Ground-level ozone (O3) is a colourless gas with a slightly sweet odour and is created by the interaction of sunlight, heat, oxides of nitrogen (NOx) and VOCs. Ozone is likely to reach unhealthy levels on hot sunny days in urban environments. Emissions from industrial facilities and electric utilities, motor vehicle exhaust, petrol and diesel vapours, and chemical solvents are some of the major sources of NOx and VOCs.
Of the six pollutants, particle pollution and ground-level ozone are the most widespread health threats. The American Environmental Protection Agency (EPA) calls these pollutants ‘criteria’ air pollutants because it regulates them by developing human health-based and/or environmentally based criteria (science-based guidelines) for setting permissible levels. The set of limits based on human health is called primary standards. Another set of limits intended to prevent environmental and property damage is called secondary standards.
Air pollution
refers to the introduction, into the atmosphere, of substances that have harmful effects on humans, other living organisms, and the environment either as solid particles, liquid droplets, or gases.
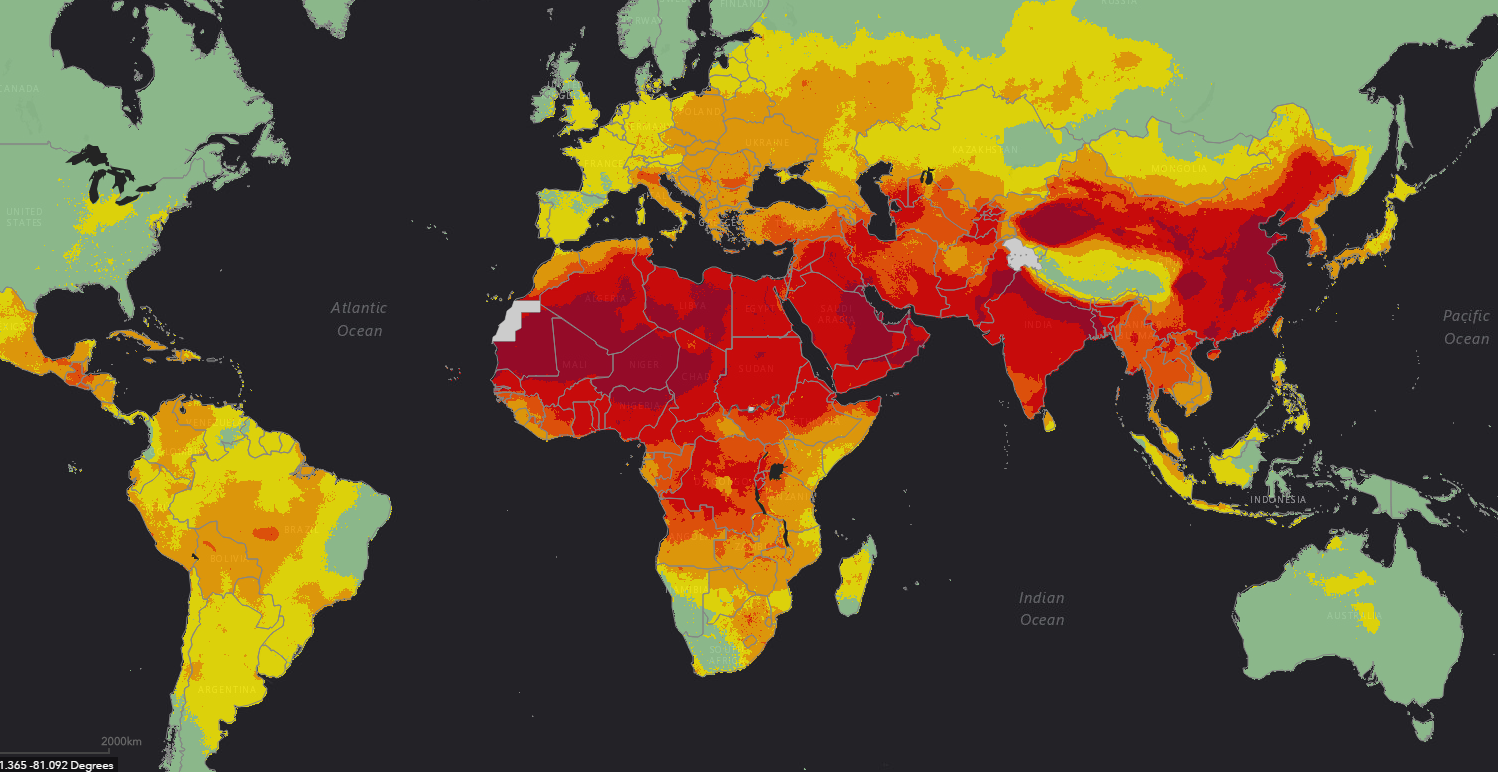
Air pollution can result from natural processes such as dust storms, forest fires, and volcanic eruptions, or from human activities such as biomass burning, vehicle emissions, mining, agriculture, and industrial processes. Natural events that result in air pollution include volcanic eruptions and veld fires, which release particles and gases into the air. Weather conditions such as wind and rain quickly disperse these pollutants and maintain the atmosphere’s natural balance.
Currently, population growth, urbanisation and industrialisation are causing more pollution than natural air cleaners can manage. Human activities that contribute to air pollution are burning/fires, industrial emissions (especially from burning fossil fuels), vehicle emissions, waste incineration and combustion on mine dumps.
Air quality in various areas of the country is affected by pollutants emitted by numerous sources. These sources include power generation activities, industrial processes, waste disposal, transportation (private and public vehicles), biomass burning, domestic fuel burning, landfill sites, wastewater treatment and agriculture.
Immediate effects of air pollution include irritation of the eyes, nose, and throat, headaches, dizziness, and fatigue. Such immediate effects are usually short-term and treatable. Sometimes the treatment is simply eliminating the person’s exposure to the source of the pollution if it can be identified. Symptoms of some diseases, including asthma, hypersensitivity pneumonitis, and humidifier fever also show up soon after exposure to some indoor air pollutants.
Air quality in a region is not just affected by the number of pollutants released into the atmosphere in that location but by other geographical and atmospheric factors. Winds can move pollutants into or out of a region and a mountain range can trap pollutants and commonly trap pollutants within a cool air mass. If the inversion lasts long enough, pollution can reach dangerous levels.
Pollutants remain over a region until they are transported out of the area by wind, diluted by air blown in from another region, transformed into other compounds, or carried to the ground when mixed with rain or snow. The conversion by ozone of hydrocarbons from automotive exhaust gases to these acids and aldehydes contributes to the irritating nature of smog.
Photochemical smog
is a mixture of pollutants that are formed when nitrogen oxides and VOCs react to sunlight and fog, creating a brown haze above cities. It tends to occur more often in summer, because that is when we have the most sunlight. It is especially prevalent in geologic basins encircled by hills or mountains. It often stays for an extended period over densely populated cities or urban areas and can build up to dangerous levels.

Primary pollutants
The two major primary pollutants, nitrogen oxides and VOCs, combine to change in sunlight in a series of chemical reactions, outlined below, to create what are known as secondary pollutants.
Secondary pollutants
The secondary pollutant that causes the most concern is ozone that forms at ground level. While ozone is produced naturally in the upper atmosphere, it is a dangerous substance when found at ground level. Many other hazardous substances are also formed, such as peroxyacetyl nitrate (PAN).
Tropospheric, or ground level ozone, is not emitted directly into the air, but is created by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOC). This happens when pollutants emitted by cars, power plants, industrial boilers, refineries, chemical plants, and other sources chemically react in the presence of sunlight.
| Primary pollutants | Secondary pollutants |
| CO | SO3 |
| CO2 | O3 |
| NO | H2SO4 |
| NO2 | HNO3 |
| SO2 | PANs |
| Most hydrocarbons | |
| Most suspended particles e.g. soot, dust |
Major sources of photochemical smog
While nitrogen oxides and VOCs are produced in nature, there are also major anthropogenic (man-made) emissions of both.
Natural sources
Natural emissions tend to be spread over large areas, reducing their effects, but man-made emissions tend to be concentrated close to their source, such as a city.
In nature, bushfires, lightning, and the microbial processes that occur in soil generate nitrogen oxides. VOCs are produced from the evaporation of naturally occurring compounds, such as terpenes, which are the hydrocarbons in oils that make them burn. Eucalyptus trees have also been found to release significant amounts of these compounds.
Man-made sources
Nitrogen oxides are produced mainly from the combustion of fossil fuels, particularly in power stations and motor vehicles. VOCs are formed from the incomplete combustion of fossil fuels, from the evaporation of solvents and fuels, and from burning plant matter – such as backyard burning and wood-burning stoves. Motor vehicles contribute 44% of VOC emissions, and area sources including petrol and solvent evaporation contribute 33%.
Smog formation
Figure 5 shows how photochemical smog is formed.
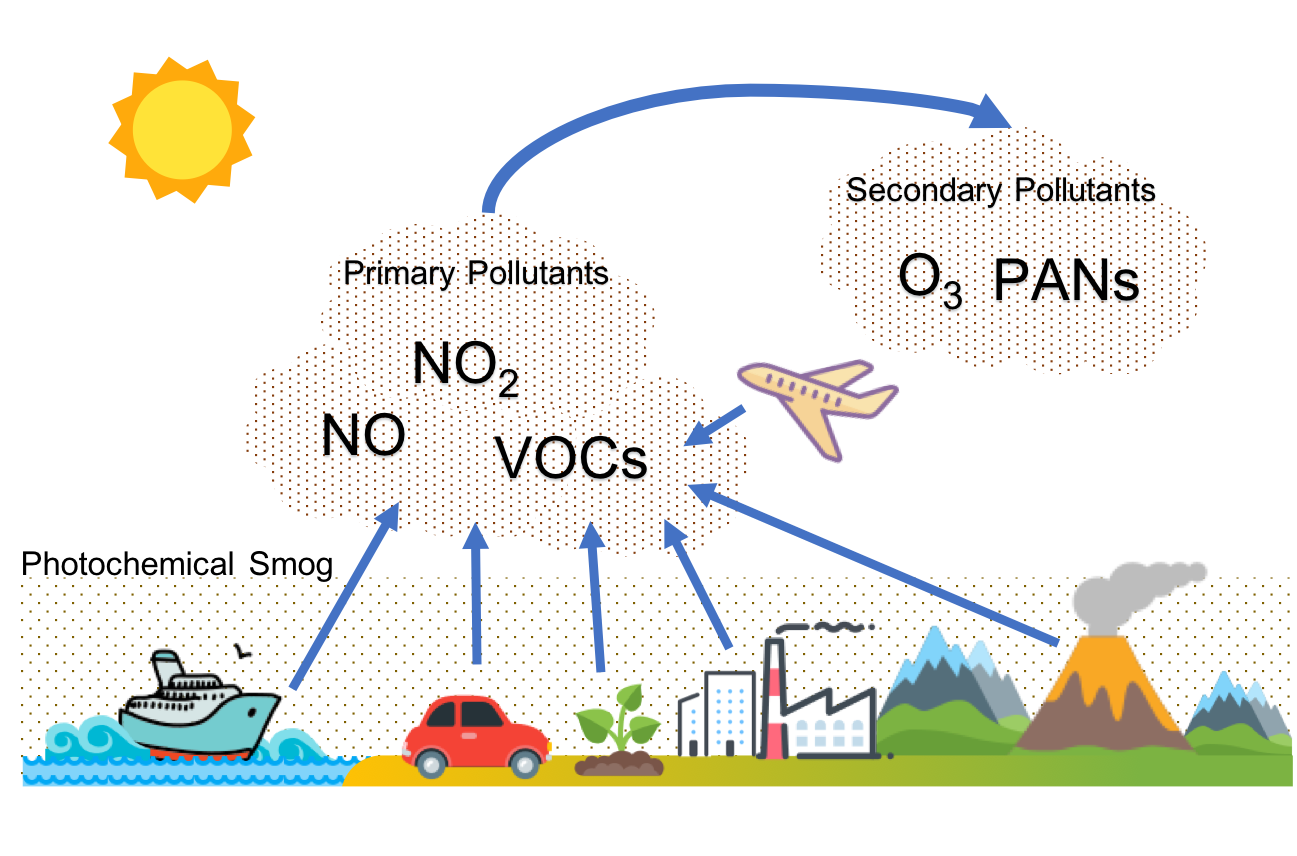
Below is a simplified explanation of the chemistry of smog formation. Nitrogen dioxide (NO2) can be broken down by sunlight to form nitric oxide (NO) and an oxygen radical (O):
- [latex]\scriptsize \displaystyle \text{N}{{\text{O}}_{\text{2}}}\text{ + sunlight }\to \text{NO + O}[/latex]
Oxygen radicals can then react with atmospheric oxygen (O2) to form ozone (O3):
- [latex]\scriptsize \displaystyle \text{O + }{{\text{O}}_{2}}\text{ }\to \text{ }{{\text{O}}_{3}}[/latex]
Ozone reacts with nitric oxide to produce nitrogen dioxide and oxygen:
- [latex]\scriptsize \displaystyle {{\text{O}}_{3}}\text{ + NO }\to \text{N}{{\text{O}}_{2}}\text{ + }{{\text{O}}_{2}}[/latex]
Harmful products, such as , are produced by reactions of nitrogen dioxide with various hydrocarbons (R), which are compounds made from carbon, hydrogen, and other substances:
- [latex]\scriptsize \displaystyle \text{N}{{\text{O}}_{2}}\text{ + R }\to \text{products such as PAN}[/latex]
The main source of these hydrocarbons is the VOCs. Similarly, oxygenated organic and inorganic compounds (ROx) react with nitric oxide to produce more nitrogen oxides:
- [latex]\scriptsize \displaystyle \text{NO + ROx }\to \text{N}{{\text{O}}_{2}}\text{ + other products}[/latex]
The significance of the presence of the VOCs in these last two reactions is vital. Ozone reacts with nitric oxide, as in reaction 3. However, when VOCs are present, nitric oxide and nitrogen dioxide are consumed as in reactions 4 and 5, allowing the build-up of ground level ozone.
Sulfurous smog
Sulfurous smog is mainly a product of burning large amounts of high sulfur coal. London was covered in smog for five days in [latex]\scriptsize \displaystyle 1952[/latex]. The smog was formed from industrial pollution and domestic fires and was responsible for [latex]\scriptsize 4000[/latex] deaths.

Clean air laws passed in [latex]\scriptsize \displaystyle 1956[/latex] have greatly reduced smog formation in the United Kingdom; however, in other parts of the world London-type smog is still very prevalent. The coal burnt in the power stations in South Africa has a high sulfur content. The main constituent of London-type smog is soot, but it also contains large quantities of fly ash, sulfur dioxide, sodium chloride and calcium sulfate particles. If concentrations are high enough, sulfur dioxide can react with atmospheric hydroxide to produce sulfuric acid, which will precipitate as acid rain.
[latex]\scriptsize \text{S}{{\text{O}}_{\text{2}}}\text{+O}{{\text{H}}^{\text{.}}}\to \text{HOS}{{\text{O}}_{\text{2}}}[/latex]
[latex]\scriptsize \text{HOS}{{\text{O}}_{\text{2}}}\text{+}{{\text{O}}_{\text{2}}}\to \text{H}{{\text{O}}_{\text{2}}}\text{+S}{{\text{O}}_{\text{3}}}[/latex]
[latex]\scriptsize \text{S}{{\text{O}}_{\text{3}}}\text{+}{{\text{H}}_{\text{2}}}\text{O}\to {{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}[/latex]
The biggest concern about photochemical smog is the effect it has on people’s health.
Note
To consolidate your knowledge of smog watch this video: The Science of Smog by TED-ED.
Climate change and air pollution
Climate change is the global variation of the earth’s climate due to natural causes and human activity caused by global warming. Global warming is accelerated by greenhouse gases caused by human activity. The main greenhouse gases are carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O). Although CO2 is the main gas contributing to climate change, it is not harmful to human health. Climate change is making air pollution worse because of ozone pollution. Ozone is formed when heat and sunlight cause chemical reactions between oxides of nitrogen and volatile organic compounds. Ozone pollution, otherwise known as smog, forms in warm weather. Warmer temperatures create conditions ideal for smog to form as they can cause the air to stagnate, keeping dirty air from leaving a given area.
Sources of air pollution
There are a variety of sources of air pollution. Some are man-made sources and some are natural.
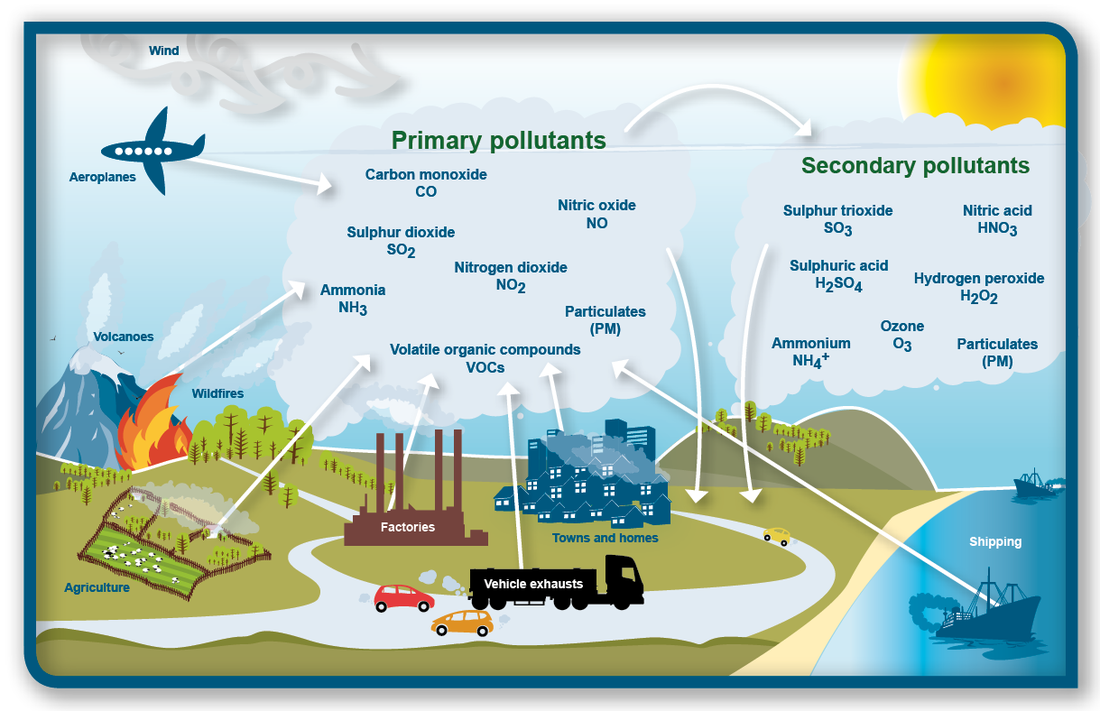
Man-made sources of air pollution
Electricity generation is by far the greatest concern with sources of air pollution in South Africa is the continued use of coal to generate electricity. South Africa generates 90% of its electricity by burning coal.
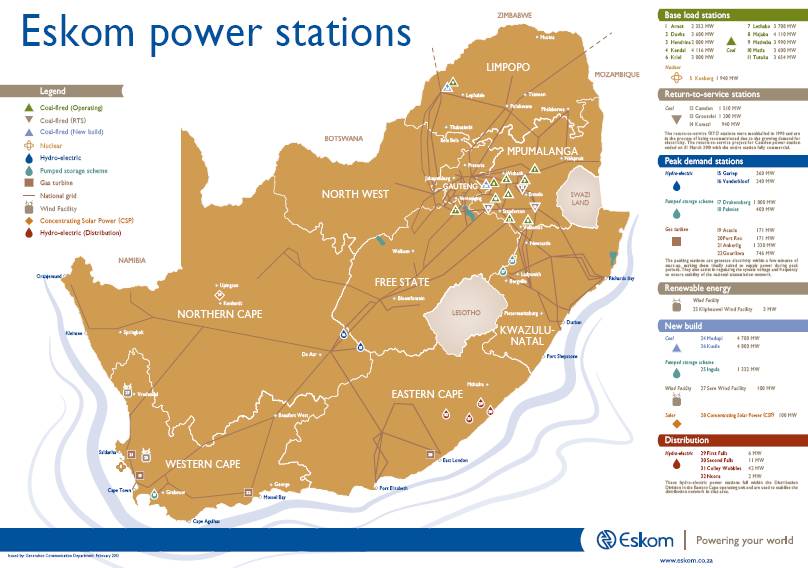
In a series of studies conducted by the World Health Organisation in 2018, it was found that Mpumalanga has some of the worst air pollution in the world. It has been calculated that air pollution from coal-fired power stations is costing the country more than R33 billion a year in health-related costs, and that the human rights of millions of South Africans are being violated with at least 2 200 people a year dying prematurely from air pollution-related illnesses like bronchitis and asthma (credit: Dr. Ranajit Sahu 2018).
In a study conducted by Dr Mike Holland in 2016, titled Health impacts of coal fired power plants in South Africa, he estimated that the following impacts are attributable to burning coal:
- 2 239 deaths per year: 157 from lung cancer; 1 110 from ischaemic heart disease, 73 from chronic obstructive pulmonary disease, 719 from strokes, and 180 from lower respiratory infection
- 2 781 cases of chronic bronchitis per year in adults
- 9 533 cases of bronchitis per year in children aged 6 to 12
- 2 379 hospital admissions per year
- 3 972 902 days of restricted activity per year
- 94 680 days of asthma symptoms per year in children aged 5 to 19
- 996 628 lost working days per year
He concluded that the total costs associated with these impacts exceed US$2 billion (approximately R28 billion) a year.
Household cooking and heating in some communities within South Africa from burning coal, wood, or paraffin to warm their homes or cook. Pollutants from the burning of wood include fine particles, nitrogen oxides, carbon monoxide, etc. The burning of paraffin produces pollutants such as nitrogen dioxide, particulate matter, carbon monoxide, formaldehyde, and sulphur dioxide. The burning of these fuels increases during winter as there is a need for heating. This results in poor air quality and an unhealthy environment. Burning wet wood produces more smoke than dry logs. This includes PM2.5 that are more harmful than bigger flakes of soot because they can penetrate deep into the respiratory system and bloodstream.
Vehicle emissions contribute to the deterioration in air quality, especially in urban areas. There has been an increase in the number of privately owned vehicles in South Africa. The increase in the number of vehicles has, as expected, resulted in an increase in fuel consumption. In urban areas, vehicle emissions may be responsible for 90% to 95% of carbon monoxide and 60% to 70% of nitrogen oxides within the atmosphere. Emissions from vehicles contribute to photochemical smog, especially in areas that experience high traffic density such as central business districts, and are responsible for the brown haze which can be seen over urban areas.
Mining processes such as mine dumps, ore handling, drilling, blasting and mine vehicle traffic travelling on unpaved roads are mainly responsible for air pollution in the form of dust, especially in open mines, which are not covered with vegetation. Blasting operations and unplanned burning also release gases such as carbon monoxide, various nitrogen oxides and sulphur dioxide. Asbestos mine dumps are a serious health hazard, and poorly managed coal mines can leak methane into the atmosphere. Coal waste contains materials that can burn on their own and produce poisonous particles and gases.
Farming also contributes to air pollution. Methane arises from animal dung, biological decay, and fermentation in the stomachs of cows and sheep. Agricultural activities such as ploughing and harvesting produce dust particles. Large-scale burning to clear agricultural fields also adds significantly to air pollution. The use of fertilisers, pesticides and herbicides also contributes to air pollution.
Refuse burning is dangerous, as refuse is a mixture of many different materials. For example, if you burn plastics or tyres, dangerous substances are given off that can harm the environment.
Natural sources of air pollution
In addition to man-made sources, large quantities of air pollutants are released from natural sources:
Veld and forest fires are common man-made and natural sources of air pollution. They spread quickly and have a large impact on the atmosphere’s chemistry, emitting pollutants such as carbon monoxide, nitrogen oxides, sulphur dioxide and tiny particles into the atmosphere. Significant quantities of pollutants are also released from natural sources such as animals, marshes, pollen, and soil.
Volcanic eruptions produce gases such as carbon dioxide, sulphur dioxide and hydrogen fluoride (HF), as well as ash particles. Eruptions release the pollutants high into the atmosphere, where they can travel long distances and cause disruptions.
Wind erosion carries dust particles that are released from soil surfaces in windy conditions, especially where there are few plants and dry soil, e.g. in deserts.
Health and environmental effects of pollution gases
Breathing in air with a high concentration of nitrous dioxide (NO2) can irritate airways in the human respiratory system. Such exposure over short periods can aggravate respiratory diseases, particularly asthma, leading to respiratory symptoms (such as coughing, wheezing or difficulty breathing), and hospital admissions. Longer exposure to elevated concentrations of NO2 may contribute to the development of asthma and potentially increase susceptibility to respiratory infections. People with asthma, as well as children and the elderly are generally at greater risk for the health effects of NO2.
The nitrate particles that result from NOx make the air hazy and difficult to see though. Ozone at ground level is a harmful air pollutant, because of its effects on people and the environment, and it is the main ingredient in ‘smog’.

Ozone is most likely to reach unhealthy levels on hot sunny days in urban environments but can still reach high levels during colder months. Ozone can also be transported long distances by wind, so even rural areas can experience high ozone levels.
Ozone in the air we breathe can harm our health. People most at risk from breathing air containing ozone include people with asthma, children, older adults, and people who are active outdoors, especially outdoor workers.
Breathing ozone can trigger a variety of health problems including chest pain, coughing, throat irritation, and airway inflammation. It also can reduce lung function and harm lung tissue. Ozone can worsen bronchitis, emphysema, and asthma, leading to increased medical care.
Ozone affects sensitive vegetation and ecosystems, including forests, parks, wildlife refuges and wilderness areas. Ozone harms sensitive vegetation during the growing season.
Ozone can damage various materials. It can cause the cracking of rubber, the reduction in tensile strength of textiles, fading of dyed fibres and cracking of paint. Ozone’s potential to damage artworks and books is of cultural importance, and some museums and libraries have taken steps to minimise this effect.
Carbon monoxide poisoning is the most common type of fatal air poisoning in many countries. Carbon monoxide is colourless, odourless and tasteless, but highly toxic. It combines with haemoglobin to produce carboxyhaemoglobin, which blocks the transport of oxygen. At concentrations above [latex]\scriptsize \displaystyle 1000\text{ ppm}[/latex] it is considered immediately dangerous and is the most immediate health hazard from running engines in a poorly ventilated space.
Take note!
ppm stands for parts per million. Ppm is commonly used as a dimensionless measure of small levels (concentrations) of pollutants in air, water, body fluids.
The effects of the major primary and secondary pollutants in smog are given in Table 2
| Pollutant | Effects |
| Nitrogen oxides |
|
| Volatile organic compounds (VOCs) |
|
| Ozone |
|
| Peroxyacetyl nitrate (PAN) |
|
Solutions to air pollution
Various air pollution control technologies and strategies are available to reduce air pollution. At its most basic level, land-use planning is likely to involve zoning and transport infrastructure planning. In most developed countries, land-use planning is an important part of social policy, ensuring that land is used efficiently for the benefit of the wider economy and population, as well as to protect the environment.
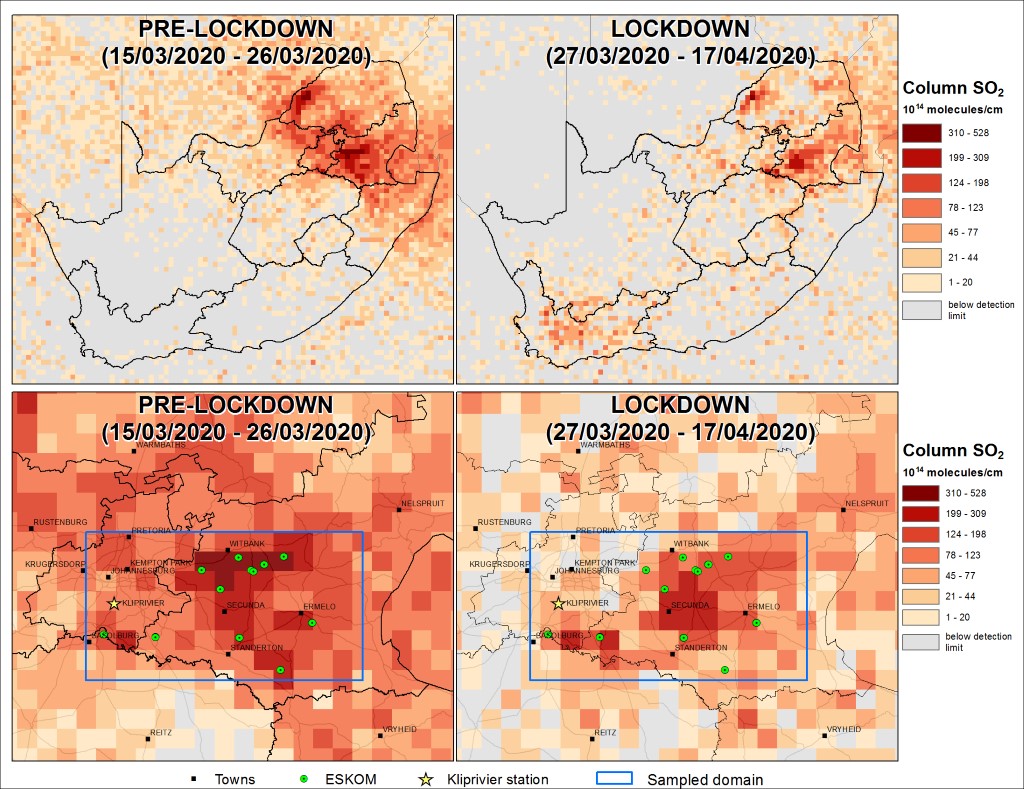
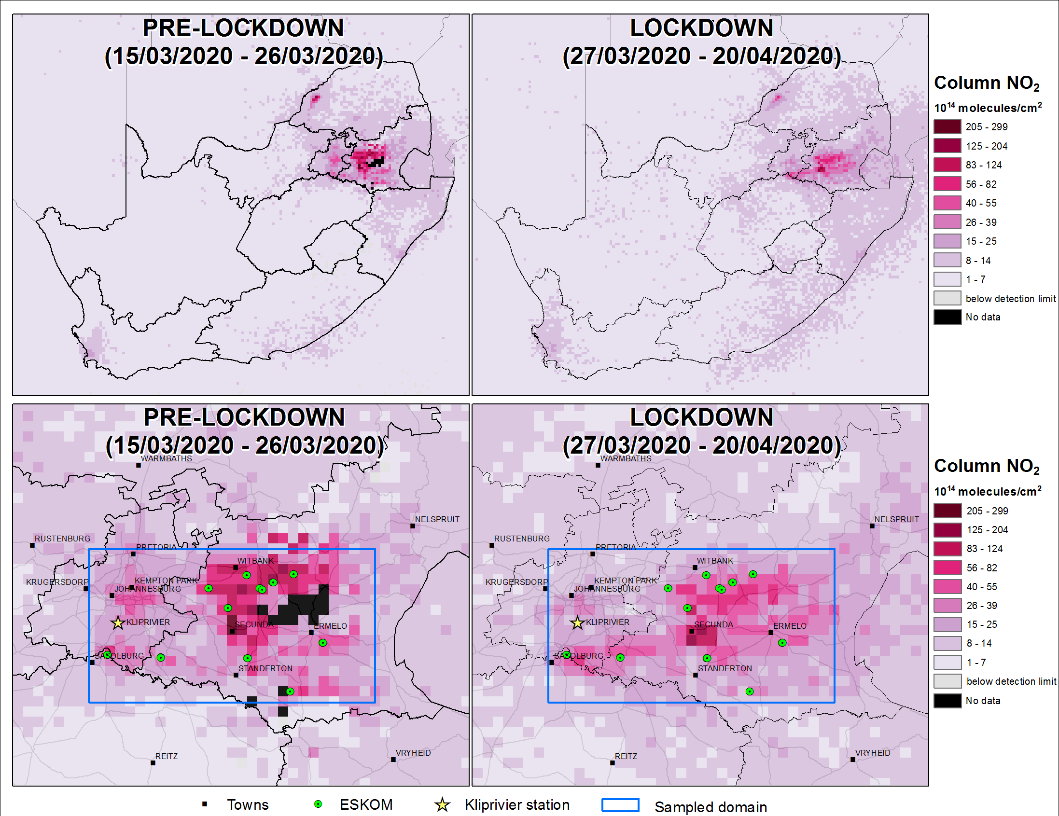
Because a large share of air pollution is caused by the combustion of fossil fuels such as coal and oil, the reduction of these fuels can reduce air pollution drastically. As can be seen in figure 10, the discontinuation of coal power stations has an immediate effect on the air quality surrounding the power stations.
Most effective is the switch to clean power sources such as wind power, solar power or hydro power which do not cause air pollution. A highly effective means to reduce air pollution is the transition to renewable energy. According to a study published in Energy and Environmental Science in 2015 the switch to 100% renewable energy in the United States would eliminate about 62 000 premature mortalities per year if no biomass were used. This would save about $600 billion in health costs a year due to reduced air pollution by 2050, or about 3.6% of the 2014 U.S. gross domestic product.
There is a timetable to decommission several coal power stations in South Africa over the next 15 years. The decommissioning of coal plants (total 28GW by 2040 and 35GW by 2050), together with emission constraints imposed by government legislation mean that coal will contribute less than 30% of the energy supplied by 2040 and less than 20% by 2050. The shortfall in generation will then be met by renewable sources.
Efforts to reduce pollution from mobile sources includes primary regulation expanding regulation to new sources (such as cruise and transport ships, farm equipment, and small petrol-powered equipment such as weed trimmers, lawn mowers and chainsaws), increased fuel efficiency (such as using hybrid vehicles), conversion to cleaner fuels or conversion to electric vehicles.
The United Kingdom and other European countries have put in place legislation which bans the sale of all new diesel and petrol vehicles from 2030. Only (new) electric and hybrid vehicles will be allowed to be sold from 2030. European cities have introduced bans or charges to drive vehicles into cities and have banned diesel lorries and trucks which do not conform to low emission standards from entering cities.
Control devices
The following items are commonly used as pollution control devices in industry and transportation. They can either destroy contaminants or remove them from an exhaust stream before being emitted into the atmosphere.
| Pollutant | Method |
| Particulate control | Mechanical collectors (dust cyclones, multicyclones) An electrostatic air cleaner is a particulate collection device that removes particles from a flowing gas using an electrostatic charge Baghouses are dust collectors consisting of a blower, dust filter, a filter-cleaning system, and a dust receptacle or dust removal system Particulate scrubbers are a form of pollution control technology |
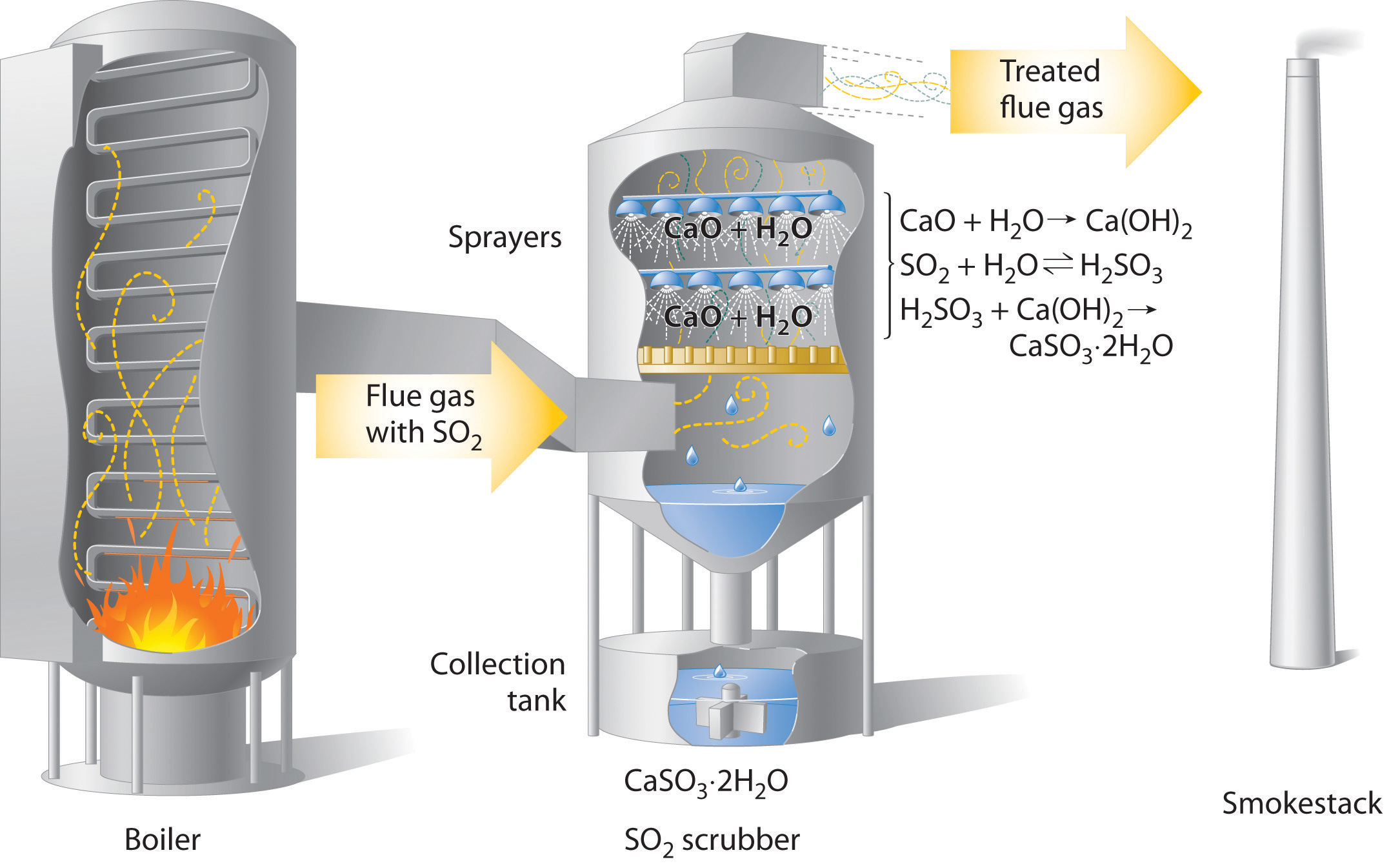
| NOx control | Low NOx burners Selective catalytic reduction Selective non-catalytic reduction NOx scrubbers Exhaust gas recirculation Catalytic converters |
| VOC | Adsorption systems, using activated carbon Flares Thermal oxidisers Catalytic converters Biofilters Absorption (scrubbing) Cryogenic condensers Vapor recovery systems |
| SO2 control | Wet scrubbers Dry scrubbers Flue-gas desulfurisation |
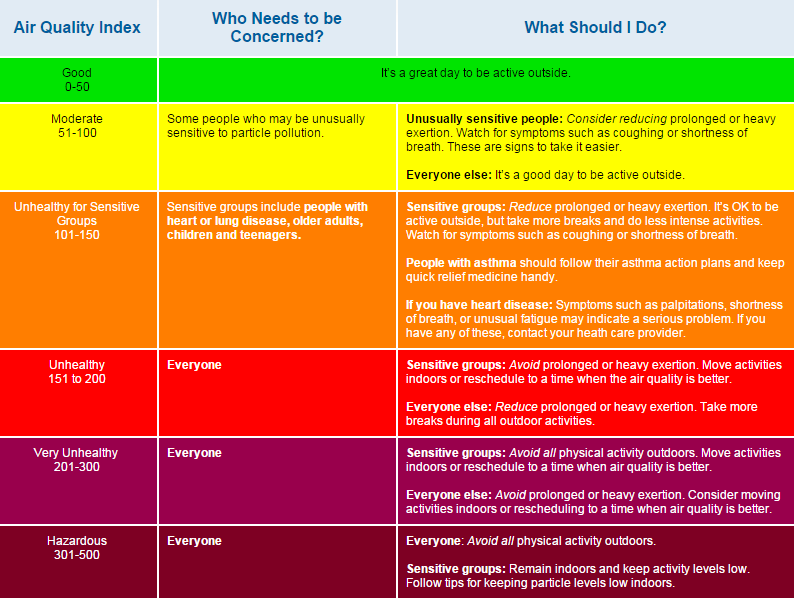
Air quality index
The Environmental Protection Agency (EPA) devised a colour coded system to identify the air quality in an area. This system has been adopted worldwide and information about air quality using this index can be found on most mobile weather apps.
Note
You can go online and look at the real-time air pollution map of South Africa.
The South African Government has an app, called SAAQIS, downloadable from the Google play store or the Apple app store which allows you to check the air quality in your area.
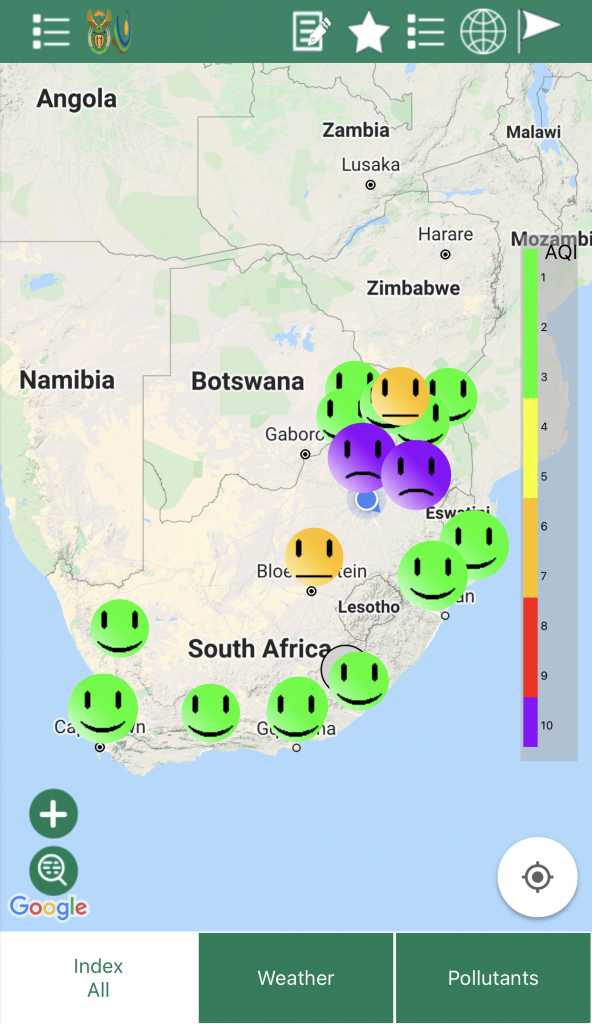
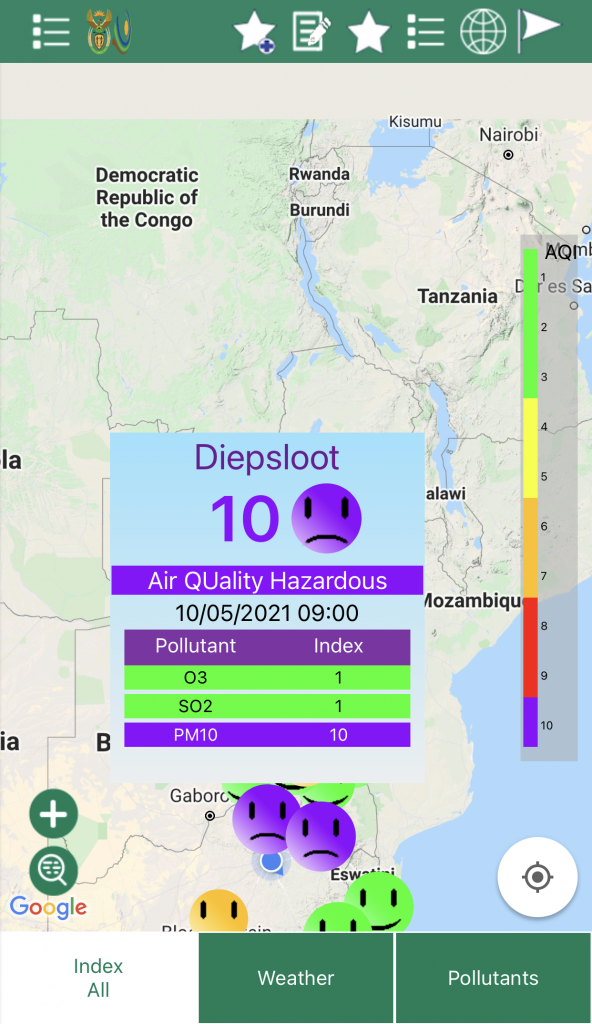
If you click on one of the faces it will then tell you the air quality at that testing station.
Note
To consolidate your knowledge of air pollution you can watch this video by National Geographic called Air Pollution 101.
Summary
In this unit you have learnt the following:
- Air pollution refers to the introduction into the atmosphere of substances that have harmful effects on humans, other living organisms, and the environment either as solid particles, liquid droplets, or gases.
- These include combustion pollutants (from burning of oil, gas, kerosene, coal, wood, and tobacco products); building materials and furnishings, products for household cleaning and maintenance, personal care, or hobbies; central heating and cooling systems and humidification devices.
- Major sources of air pollution are gas emissions from fossil-fuelled vehicles and their reaction products (CO, NOx, Ozone) as well as particulate matter, SO2, and VOCs.
- Smog is a mixture of air pollutants (sulfur dioxide, nitrogen oxides, ozone, and particulates) that often form over urban areas because of fossil fuel combustion. The term was coined from the terms ‘smoke’ and ‘fog’ referring to a brownish haze that pollutes the air, greatly reducing visibility and making it difficult for some people to breathe.
- Photochemical smog is a mixture of pollutants that are formed (mostly during the hot summer months) when nitrogen oxides and volatile organic compounds (VOCs) react to sunlight, creating a brown haze above cities.
- Photochemical smog is formed from the reactions of natural and man-made emissions of nitrogen oxides and VOCs.
- Air pollution is a serious problem in many cities and continues to harm human health. Ground-level ozone, sulphur dioxide, nitrogen dioxide and carbon monoxide are especially harmful for senior citizens, children, and people with heart and lung conditions such as emphysema, bronchitis, and asthma.
Unit 2: Assessment
Suggested time to complete: 20 minutes
Please answer the following multiple-choice questions by giving the most correct answer
- What are the two main causes of air pollution?
- Man-made and natural sources
- Natural resources and waste disposal
- Natural sources and climate change
- Man-made and natural resources
- Which two pollutants result in man-made air pollution.
- Nitrogen and carbon dioxide
- Carbon monoxide and nitrogen
- Oxygen and carbon dioxide
- Carbon monoxide and nitrogen oxide
- What are the causes of man-made air pollution and natural air pollution respectively?
- Waste incinerators and volcanic eruptions
- Swimming and wind erosion
- Smoking and forest fires
- Hurricanes and power plant emissions
- a and c
- Which three effects of air pollution harm the environment.
- Destroy ecosystem, acid rain destroys habitats and climate changes
- Corrode buildings, affect photosynthesis, and cause poor visibility
- Destroy plants, destroy ecosystem, and cause soil erosion
- Cause climate changes, cause natural disasters and destroys habitats
- What is an example of natural air pollution?
- Acid rain
- Vehicle emission of harmful gases
- Volcanic eruption
- Burning fuels
- Which of the following sources create pollutants that are responsible for smog?
- Incinerators
- Emissions from vehicles
- Both incinerators and emissions from vehicles
- None of the above
- Which of the following are secondary air pollutants?
- PANs
- Ozone
- Carbon monoxide
- a and b
- Which of the following are particulate pollutants?
- Ozone
- Radon
- Fly ash
- Ethylene
- Which of the following can be classified as a primary pollutant?
- Sulfur dioxide
- Carbon dioxide
- Hydrogen fluoride
- a and b
- Which of the following statements is true about smog?
- Smog is made up of fog.
- Smog is made from smoke and ozone.
- Smog is made up of water vapour.
- Smog is made up of fog and nitrogen dioxide.
- What type of precautions should be taken to reduce ozone pollution?
- Drive less.
- Only fill your vehicle with fuel when the temperature is cooler.
- Use public transport where possible.
- All the above.
- Which of the following statements is true about the air quality index?
- It indicates the colour of the air.
- It predicts ozone levels in your area.
- It determines the intensity of sound pollution.
- It estimates air pollution, mainly sulphur content, in the air.
- Which of the following diseases are caused by smog?
- Heart disease.
- Bronchitis
- Breathing problems
- All the above
- Smoke, fumes, ash, dust, nitric oxide, and sulphur dioxide are the main sources of ________.
- primary pollutants
- secondary pollutants
- bio-degradable pollutants
- None of the above
- Which of the following industries plays a major role in polluting air and increasing air pollution?
- Manufacture of gases industries
- Electricity generation
- Electrical appliances and electrical goods industries
- All the above
- Increased levels of air pollution results in _______.
- soil erosion
- global warming
- respiratory problems
- b and c.
The full solutions can be found at the end of the unit.
Unit 2: Solutions
Unit 2: Assessment
- a
- d
- e
- c
- c
- c
- d
- c
- d
- d
- d
- b
- d
- a
- a
- d
Media Attributions
- Fig 1 © WHO is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 2 © Libretext is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 3 © WHO is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Smog over Almaty © Igors Jefimovs is licensed under a CC BY (Attribution) license
- Fig 5 © Liweichao.vivian, CC BY-SA 4.0 , via Wikimedia Commons is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 6 is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 7 © Mr G Science is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 8 © Eskom is licensed under a Public Domain license
- Fig 9 © EPA is licensed under a All Rights Reserved license
- Fig 10 © CSIR is licensed under a All Rights Reserved license
- Fig 10 © CSIR is licensed under a All Rights Reserved license
- Fig 11 © DHET is licensed under a CC BY (Attribution) license
- Fig 13 © Lumen is licensed under a CC BY (Attribution) license
- Fig 14 © EPA is licensed under a Public Domain license
- Fig 12 © Utah State University is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- fig 15 © DHET is licensed under a CC BY (Attribution) license
- Fig 16 © DHET is licensed under a CC BY (Attribution) license
- Fig 17 © DHET is licensed under a CC BY (Attribution) license
resulting from the influence of human beings on nature
volatile organic compounds are gases emitted from certain solids or liquids, such as paints, cleaning supplies, pesticides, building materials, furnishings, adhesives, and permanent markers
emissions that directly cause air pollution in the form in which they are released at the source
forms from primary pollutants emitted from sources reacting with molecules in the atmosphere
fog or haze intensified by smoke or other atmospheric pollutant
polluted rain caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air; these substances can rise very high up into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants called acid rain
the presence in or introduction into the air of a substance which has harmful or poisonous effects
haze in the atmosphere accompanied by high levels of ozone and nitrogen oxides, caused by the action of sunlight on pollutants
peroxyacyl nitrates are powerful respiratory and eye irritants present in photochemical smog
substances capable of causing cancer
