Mechanical Properties of matter: State, analyse and explain change of state due to molecular forces
Unit 1: Thermal properties of solids and liquids
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Describe and identify intermolecular bonding.
- Describe and identify intramolecular forces.
- Describe and identify hydrogen bonding.
- Describe and identify van de Waal’s forces.
What you should know
Before you start this unit, make sure you can:
- Identify the differences between solids, liquids, and gases. Refer to level 2 subject outcome 5.1 unit 1 to revise this.
- Explain covalent and ionic bonding. Refer to level 2 subject outcome 5.4 unit 3 to revise this.
- Understand electronegativity and polarity. Refer to level 3 subject outcome 5.2 unit 2 to revise this.
- Define polarity and polar molecules. Refer to level 3 subject outcome 5.2 unit 5 to revise this.
- Explain molecular shape. Refer to level 3 subject outcome 5.2 unit 4 to revise this.
Introduction
In this unit you will learn about different types of intermolecular and intramolecular bonding. Intermolecular refers to the bonding between atoms to form molecules and intramolecular bonding forms when molecules stick together because of electrostatic forces.
Note
The prefix ‘inter’ means between or amongst.
The prefix ‘intra’ means within.
Inter molecular and intramolecular forces
are the forces between atoms in a molecule, commonly called bonding. There are three types of intramolecular forces: ionic bonding, covalent bonding, and metallic bonding. Intramolecular forces are those within the molecule that keep the molecule together, for example in water, the intramolecular force is the bond between the atoms of hydrogen and oxygen.
are forces that act between the particles of a substance, regardless of whether these particles are molecules, atoms, or ions. Intermolecular forces are electrostatic forces of attraction that may exist between the atoms and molecules of a substance. These forces hold particles close together. As the kinetic energy of the particles increases, these forces can be overcome and the distance between particles will increase.
As we learnt in level 2 subject outcome 5.1 unit 1, particles in a solid are tightly packed together and often arranged in a regular pattern; in a liquid, they are close together with no regular arrangement; in a gas, they are far apart with no regular arrangement. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; in a liquid, they move past each other but remain in essentially constant contact; in a gas, they move independently of one another except when they collide.
The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The phase in which a substance exists depends on the extent of its intermolecular forces and the kinetic energy of its molecules.
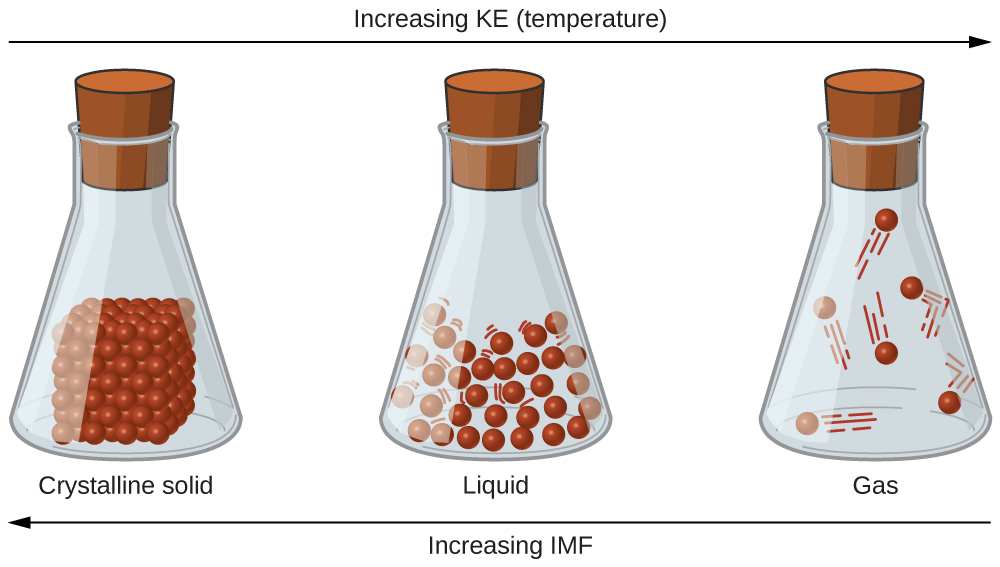
Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together, thereby forming bubbles of vapour within the liquid. Similarly, solids melt when the molecules acquire enough thermal energy to overcome the intermolecular forces that lock them into place in the solid.
Intermolecular forces come from the interaction between positively and negatively charged species or positively and negatively charged poles on molecules. Like covalent and ionic bonds, intermolecular interactions are the sum of both attractive and repulsive components. Because electrostatic interactions fall off rapidly with increasing distance between molecules, intermolecular interactions are most important for solids and liquids, where the molecules are close together. These interactions become important for gases only at very high pressures. We can also liquefy many gases by compressing them if the temperature is not too high. The increased pressure brings the molecules of a gas closer together, such that the attractions between the molecules become strong relative to their KE. Consequently, they form liquids. Butane is the fuel used in disposable lighters and is a gas at standard temperature and pressure. Inside the lighter’s fuel compartment, the butane is compressed to a pressure that results in its condensation to the liquid state.
When gaseous water is cooled sufficiently, the attractions between H2O molecules will be capable of holding them together when they come into contact with each other; the gas condenses, forming liquid H2O.

Intermolecular forces are a microscopic property which affects the macroscopic behaviour of substances. If a substance has weak intermolecular forces, then it will evaporate easily. Substances with weak intermolecular forces also have low and do not rise as far up in narrow tubes as substances with strong intermolecular forces. Boiling points are lower for substances with weak intermolecular forces. Substances are more likely to be soluble in liquids with similar intermolecular forces.
Polarity and intermolecular forces
As we learnt in subject outcome 5.2 unit 5, molecules can be either polar or non-polar. A polar molecule is one in which there are polar bonds (there is a difference in electronegativity between the atoms in the molecule, such that the shared electron pair spends more time close to the atom that attracts it more strongly) and the shape of the molecule is asymmetrical. The result is that one end of the molecule will have a slightly positive charge ([latex]\scriptsize \displaystyle {{\delta }^{+}}[/latex]), and the other end will have a slightly negative charge ([latex]\scriptsize \displaystyle {{\delta }^{-}}[/latex]). The molecule is said to be a .
Polar bonds form between atoms, and polar molecules are formed when the bond forms a . A dipole moment arises in any system when there is a separation of charge. This means they can form in ionic bonds as well as in covalent bonds. Dipole moments occur due to the difference in electronegativity between two chemically bonded atoms.
A bond dipole moment is a measure of the polarity of a chemical bond between two atoms in a molecule. It involves the concept of an electric dipole moment, which is a measure of the separation of negative and positive charges in a system.
It is important to be able to recognise whether the molecules in a substance are polar or non-polar because this will determine what type of intermolecular forces there are and the polarity of molecules affects properties such as solubility, melting points and boiling points.
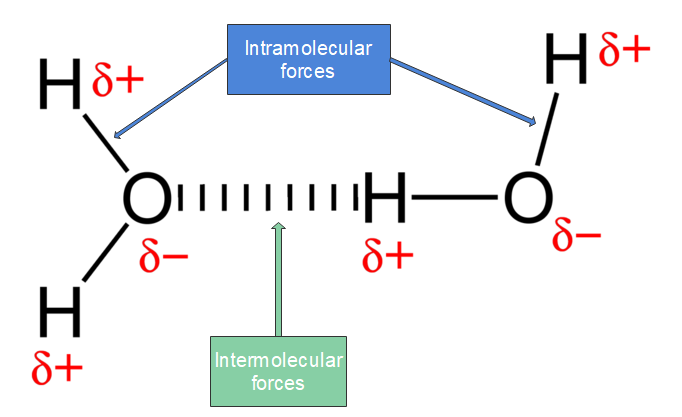
Water is polar because of polar bonding and the bent shape of the molecule. The shape means most of the negative charge is on the oxygen side of the molecule and the positive charge is on the hydrogen side of the molecule.
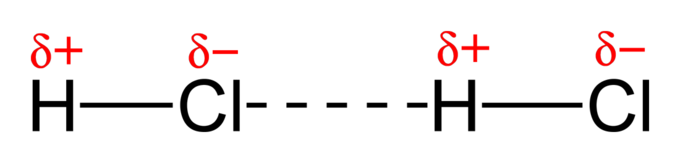
Because chlorine is more electronegative than hydrogen, chlorine in the HCl molecule polarises electron density towards itself. Therefore, the H−Cl molecule is a polar covalent molecule, in which the electronegative chlorine atom strongly polarises electron density.
A dipole molecule is a molecule that has two (di) poles. One end of the molecule is slightly positive and the other is slightly negative. It is important to remember that just because the bonds within a molecule are polar, the molecule itself may not necessarily be polar. The shape of the molecule may also affect its polarity. The interaction between the two dipoles is an attraction rather than a bond because no electrons are shared between the two molecules.
Under appropriate conditions, the attractions between all gas molecules will cause them to form liquids or solids. This is due to intermolecular forces.
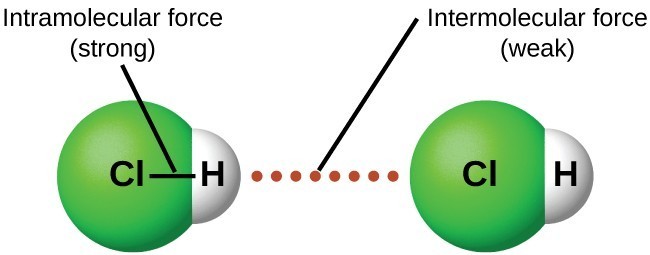
The strengths of these attractive forces vary widely, though usually the intermolecular forces (IMFs) between small molecules are weak compared to the intramolecular forces that bond atoms together within a molecule.
Activity 1.1: Investigate evaporation and to determine the relationship between evaporation and intermolecular forces
Time required: Up to an hour
What you need:
- [latex]\scriptsize \displaystyle 20\text{ ml}[/latex] of ethanol
- [latex]\scriptsize \displaystyle 20\text{ ml}[/latex] water
- [latex]\scriptsize \displaystyle 20\text{ ml}[/latex] nail polish remover (acetone)
- [latex]\scriptsize \displaystyle 20\text{ ml}[/latex] methylated spirits
- [latex]\scriptsize 4[/latex] shallow basins, saucers, or side plates
- a permanent marker
- a stopwatch/timer
What to do:
- Copy the table below into your notebook to record your results.
- Place [latex]\scriptsize \displaystyle 20\text{ ml}[/latex] of each substance required in separate identical evaporating dishes.
- Carefully move each dish to a warm and sunny spot.
- Mark the level of liquid in each dish using a permanent marker.
- Observe each dish every minute and note which liquid evaporates fastest.
| Level after (mins): | |||||
| Substance | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize 4[/latex] | [latex]\scriptsize 5[/latex] |
| Water | |||||
| Ethanol | |||||
| Acetone | |||||
| Methylated spirits | |||||
You do not need to measure the level of the liquid, but rather just write how much the level had dropped (e.g. for water you might write ‘did not notice any decrease in the level’ or for ethanol you might write ‘almost all the liquid had evaporated’).
What did you find?
Water takes the longest time to evaporate, and the acetone takes the least amount of time.
Water has strong intermolecular forces (hydrogen bonds). Ethanol ([latex]\scriptsize \displaystyle \text{C}{{\text{H}}_{3}}\text{C}{{\text{H}}_{2}}\text{OH}[/latex]) and methylated spirits, which is a mixture of ethanol with some methanol ([latex]\scriptsize \displaystyle \text{C}{{\text{H}}_{3}}\text{OH}[/latex]) both have hydrogen bonds, but these are slightly weaker than the hydrogen bonds in water. Acetone ([latex]\scriptsize \displaystyle \text{C}{{\text{H}}_{3}}\text{COC}{{\text{H}}_{3}}[/latex]) has dipole-dipole forces only and so evaporates quickly.
Substances with weaker intermolecular forces evaporate faster than substances with stronger intermolecular forces.
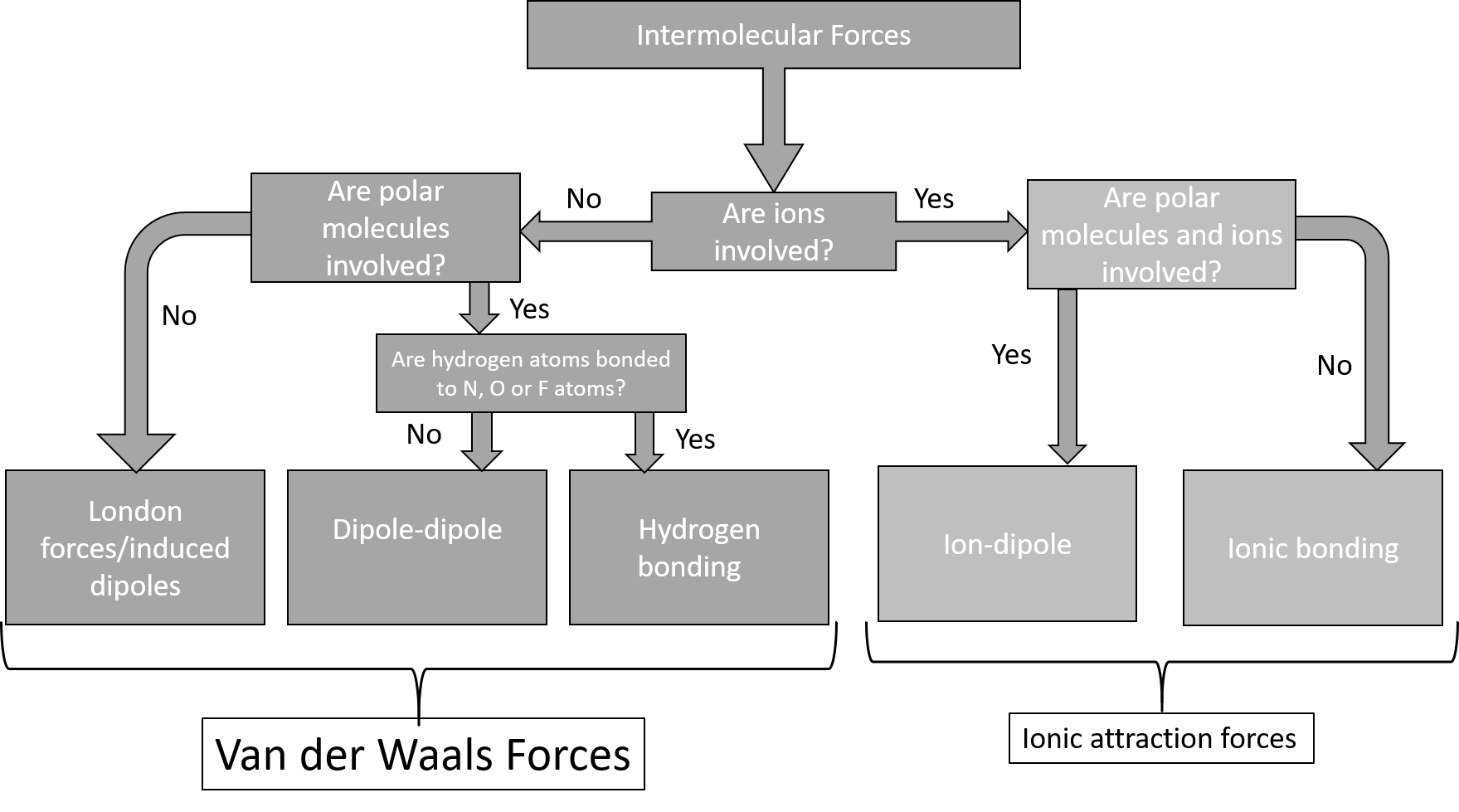
Types of intermolecular forces
It is important to remember that just because the bonds within a molecule are polar, the molecule itself may not necessarily be polar. As we learnt in subject outcome 5.2 unit 5, the shape of the molecule may also affect its polarity. Molecules will be polar if the bonds are polar, and the shape of the molecule is not symmetrical. So linear and tetrahedral shaped molecules are not polar but bent and triagonal pyramidal are.
Van der Waals forces
only occur in covalently bonded molecules, and they are weaker than normal covalent and ionic bonds. They are all short-range forces and hence only interactions between the nearest particles need to be considered (instead of all the particles). Van der Waals attraction is greater if the molecules are closer.
- : this type of intermolecular force exists between an ion and a dipole (polar) molecule. A positive ion will be attracted to the negative pole of the polar molecule, while a negative ion will be attracted to the positive pole of the polar molecule. This can be seen when sodium chloride (NaCl) dissolves in water. The positive sodium ion (Na+) will be attracted to the slightly negative oxygen atoms in the water molecule, while the negative chloride ion (Cl–) is attracted to the slightly positive hydrogen atoms. These intermolecular forces weaken the ionic bonds between the sodium and chloride ions so that the sodium chloride dissolves in the water.
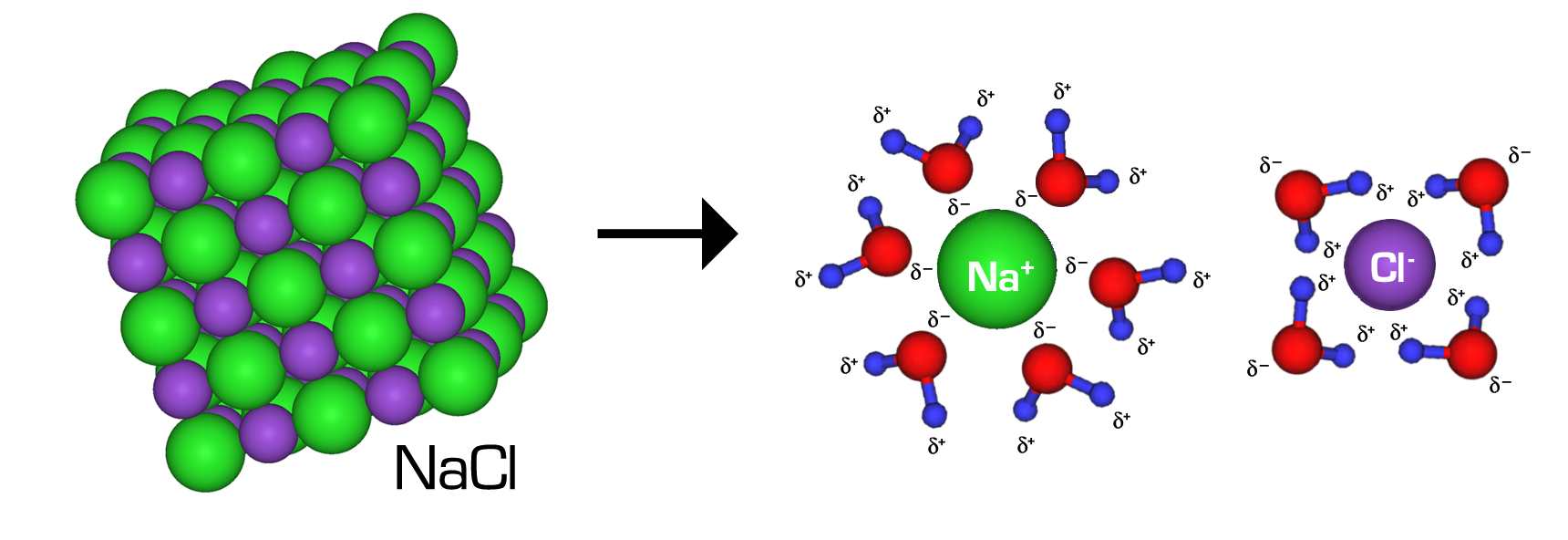
Figure 6: Ion-dipole forces in a sodium chloride solution - : when one dipole molecule comes into contact with another dipole molecule, the positive pole of the one molecule will be attracted to the negative pole of the other, and the molecules will be held together in this way. Examples of materials/substances that are held together by dipole-dipole forces are [latex]\scriptsize \displaystyle \text{HCl}[/latex], [latex]\scriptsize \displaystyle \text{S}{{\text{O}}_{\text{2}}}[/latex] and [latex]\scriptsize \displaystyle \text{C}{{\text{H}}_{\text{3}}}\text{Cl}[/latex].
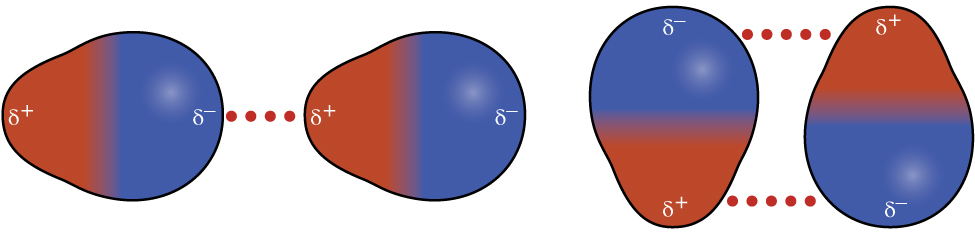
Figure 7: Two arrangements of polar molecules, such as HCl, that allow an attraction between the partial negative end of one molecule and the partial positive end of another - : this force is similar to an ion-dipole force, and exists between ions and non-polar molecules. The ion induces a dipole in the non-polar molecule leading to a weak, short-lived force which holds the particles together. These forces are found in haemoglobin (the molecule that carries oxygen around your body). Haemoglobin has [latex]\scriptsize \displaystyle \text{F}{{\text{e}}^{{\text{2+}}}}[/latex] ions. Oxygen ([latex]\scriptsize \displaystyle {{\text{O}}_{\text{2}}}[/latex]) is attracted to these ions by ion-induced dipole forces.
- : this force is also known as a London force, or a dispersion force. In non-polar molecules the electronic charge is usually evenly distributed but it is possible that at a particular moment in time, the electrons might not be evenly distributed (remember that the electrons are always moving in their orbitals). The molecule will become a temporary dipole; opposite ends of the molecules have a slight charge, either positive or negative. When this happens, molecules that are next to each other attract each other very weakly. These forces are found in the halogens (e.g. F2 and Cl2) and in other non-polar molecules such as carbon dioxide and carbon tetrachloride.
.
All covalent molecules have induced dipole forces. For non-polar covalent molecules, these forces are the only intermolecular forces. For polar covalent molecules, dipole-dipole forces are found in addition to the induced dipole forces. When the noble gases condense, the intermolecular forces that hold the liquid together are induced dipole forces.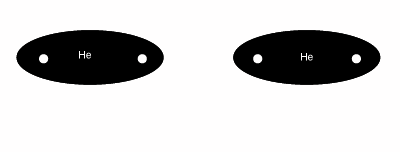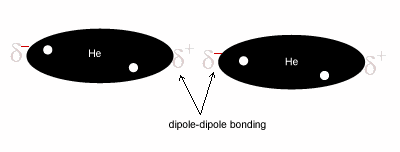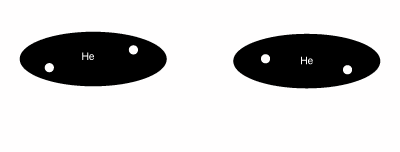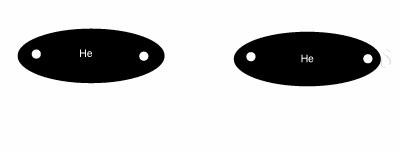
Figure 8: The result of the development of many temporary dipoles is a series of brief dispersion forces .
Induced dipole forces are very weak because the charges that generate these forces are not long lasting and disappear as quickly as they are formed. Large molecules tend to have a greater surface area for dispersion forces to form and therefore the forces acting between the molecules, although weak, are slightly stronger than the forces between smaller molecules. - : this type of force occurs when a dipole molecule induces a dipole in a non-polar molecule. This is like an ion-induced dipole force. An example of this type of force is chloroform (CHCl3) and carbon tetrachloride (CCl4).
Take note!
The three forces (dipole-dipole forces, dipole-induced dipole forces and induced dipole forces) are collectively known as van der Waals forces.
- : this is a special type of dipole-dipole force, that develops between molecules containing a hydrogen atom covalently bonded to another highly electronegative atom (F, O or N).
.
Water molecules for example, are held together by hydrogen bonds between the hydrogen atom of one molecule and the oxygen atom of another. Hydrogen bonds are a relatively strong intermolecular force and are stronger than other dipole-dipole forces. It is important to note however, that hydrogen bonds are weaker than the covalent and ionic bonds that exist between atoms. As a result of hydrogen bonding, molecules such as water (H2O), ammonia (NH3) and hydrogen fluoride (HF) have much higher boiling points and melting points than expected for such small molecules.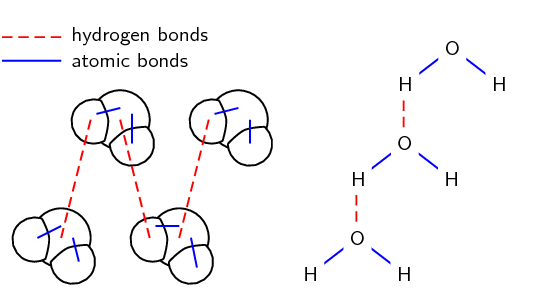
Figure 9: Water is a covalently bonded molecule held together by intramolecular forces, shown with the blue lines; the hydrogen bonds between the molecules of water are shown with the red dashed lines
Take note!
It is important to note that a hydrogen bond is not an actual bond, it is an intermolecular force.
Note
To consolidate your understanding of intermolecular forces you can watch this video by Snaprevise: Intermolecular forces.
Example 1.1
Which intermolecular forces are found in carbon tetrachloride (CCl4)?
Solution
Step 1: Think about what you know about the molecule
Carbon has an electronegativity of [latex]\scriptsize \displaystyle 2.5[/latex]. Chlorine has an electronegativity of [latex]\scriptsize \displaystyle 3.0[/latex]. The electronegativity difference between a carbon atom and a chlorine atom is [latex]\scriptsize 3.0-2.5=0.5[/latex]. Therefore the bonds will be polar.
Step 2: Is the molecule polar?
We know that the bond between carbon and chlorine is polar, but the molecule of CCl4 is nonpolar because it has a symmetrical tetrahedral shape.
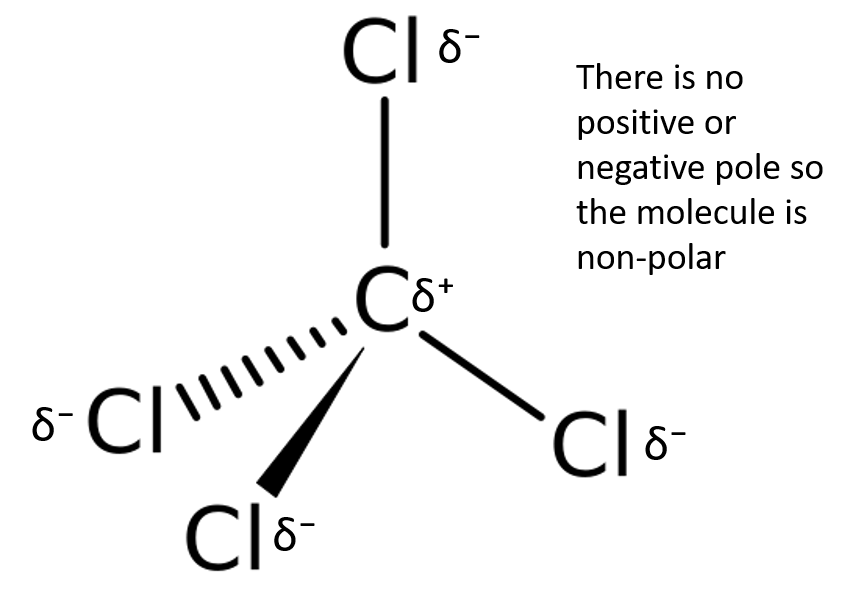
Step 3: Decide which intermolecular forces are found in CCl4
Carbon tetrachloride is non-polar and so the only kind of force that can exist is induced dipole forces (or London forces).
Here is a tabulated summary of the intramolecular and intermolecular forces.
| Intramolecular forces | Intermolecular forces |
| Forces between atoms | Forces between molecules |
| Strong forces | Weak forces |
| Short distance between atoms | Larger distances than in intramolecular forces |
| Covalent Ionic Metallic |
Ion-dipole London forces Dipole-dipole Hydrogen bonds |
Exercise 1.1
- Give one word or term for each of the following descriptions:
- The attractive force that exists between molecules.
- A molecule that has an unequal distribution of charge.
- Thato states that van der Waals forces include ion-dipole forces, dipole-dipole forces, and induced dipole forces.
.
Simphiwe states that van der Waals forces include ion-dipole forces, ion-induced dipole forces and induced dipole forces.
.
Thabile states that van der Waals forces include dipole-induced dipole forces, dipole-dipole forces, and induced dipole forces.
.
Who is correct and why? - Theo and Bongani are arguing about which molecules have which intermolecular forces. They have drawn up the following table:
Compound Type of force Potassium iodide in water (KI(aq)) dipole-induced dipole forces Hydrogen sulfide (H2S) induced dipole forces Helium (He) ion-induced dipole forces Ammonia (NH3) Dipole-dipole forces - Theo says that hydrogen sulfide is non-polar and so has induced dipole forces. Bongani says hydrogen sulfide is polar and has dipole-dipole forces. Who is correct and why?
- Bongani says that helium (He) is an ion and so has ion-induced dipole forces. Theo says helium is non-polar and has induced dipole forces. Who is correct and why?
- They both agree on the rest of the table. However, they have not got the correct force for potassium iodide in water (KI(aq)). What type of force exists in this compound?
The full solutions can be found at the end of the unit.
Summary
In this unit you have learnt the following:
- Intermolecular forces occur between molecules.
- Intermolecular forces are the forces that hold the atoms in molecules together.
- The type of intermolecular force in a substance depends on the nature of the molecules.
- Polar molecules have an unequal distribution of charge, meaning that one part of the molecule is slightly positive, and the other part is slightly negative. The molecule is said to be a dipole.
- Non-polar molecules have an equal distribution of charge.
- There are five types of intermolecular forces: ion-dipole forces, ion-induced-dipole forces, dipole-dipole forces, dipole-induced dipole forces and induced dipole forces.
- Ion-dipole forces exist between ions and polar (dipole) molecules. The ion is attracted to the part of the molecule that has an opposite charge to its own.
- Ion-induced dipole forces exist between ions and non-polar molecules. An ion induces a temporary dipole in the non-polar molecule.
- Dipole-dipole forces exist between two polar (dipole) molecules.
- Dipole-induced dipole forces exist between a polar molecule and a non-polar molecule.
- Induced dipole forces exist between two non-polar molecules.
- Dipole-dipole forces, dipole-induced dipole forces and induced dipole forces are collectively called van der Waals forces.
- Hydrogen bonds are a type of dipole-dipole force that occurs when a hydrogen atom is bonded to a highly electronegative atom (oxygen, fluorine, nitrogen). The hydrogen atom on one molecule is attracted to the electronegative atom on a second molecule.
- Intermolecular forces affect the properties of substances.
- In general, substances with larger molecules have stronger intermolecular forces than substances with smaller molecules.
Unit 1: Assessment
Suggested time to complete: 25 minutes
- Which of these is not an intermolecular force?
- Dipole-dipole forces
- London dispersion forces
- Covalent bonding
- Hydrogen bonding
- Which of the following type of force is the strongest intermolecular force?
- London dispersion force
- Hydrogen bonding
- Ionic bonding
- Dipole-dipole
- What type of intermolecular force is present in Hydrogen fluoride (HF)?
- Dipole-dipole
- Dipole-induced dipole
- Hydrogen bonding
- Covalent bonding
- Which type of intermolecular force is present in chlorine (Cl2)?
- London dispersion force
- Hydrogen bonding
- Ionic bonding
- Dipole-dipole
- Hydrogen bonding is a special type of what force?
- Dipole-dipole
- Dipole-induced dipole
- Hydrogen bonding
- Covalent bonding
For the following questions state which intermolecular forces are present in:
- Helium (He)
- Methane (CH3)
- Carbon dioxide (CO2)
The full solutions can be found at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- .
- Intermolecular force
- A polar molecule
- Thabile is correct. Van der Waals forces are the only forces that can exist with covalent molecules and so including either ion-dipole or ion-induced dipole forces is not correct.
- .
- Theo is correct. Hydrogen sulfide is nonpolar. Although it has an asymmetrical molecular geometry, the entire molecule is non-polar due to the absence of any polar bonds.
- Theo is correct. Helium is a noble gas and so exists as single atoms, not as a compound. Helium is non-polar and so has induced-dipole forces.
- KI(aq) has potassium and iodide ions in water. Water is a polar molecule, so the type of force must be ion-dipole.
Unit 1: Assessment
- c
- b
- c
- a
- a
- Helium is non-polar covalent molecule. It is linear and symmetrical. So the type of intermolecular force is London dispersion force.
- Methane is a non-polar covalent molecule. It is tetrahedral and symmetrical, so the type of intermolecular force is London dispersion forces/induced dipole forces.
- Carbon dioxide is a nonpolar covalent molecule. It is linear and symmetrical. So the type of intermolecular force is London dispersion force/induced dipole force
Media Attributions
- Fig 1 © Opentext is licensed under a CC BY (Attribution) license
- Fig 2 © Peter Griffin is licensed under a CC0 (Creative Commons Zero) license
- Fig 3 © DHET is licensed under a CC BY (Attribution) license
- Fig 4 © Boundless Learning is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 5 © Opentext is licensed under a CC BY (Attribution) license
- Fig 6 © Ahazard.sciencewriter is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 7 © Opentext is licensed under a CC BY (Attribution) license
- Fig 8 (a) © dynamic science is licensed under a All Rights Reserved license
- Fig 9 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 10 © DHET is licensed under a CC BY (Attribution) license
- Fig 11 © DHET is licensed under a CC BY (Attribution) license
forces that exist between atoms within a molecule
forces that exist between molecules
the tendency of fluid surfaces to shrink into the minimum surface area possible
a molecule that consists of two oppositely charged sides
arises in any system in which there is a separation of charge
weak, short-range electrostatic attractive forces between uncharged molecules from the interaction of permanent or temporary dipole moments
interaction involving a molecule that is a permanent dipole and an ion
interaction which occurs between molecules which are permanent dipoles
a weak attraction that results when an ion interacts with a non-polar molecule
when a molecule that is not polar, becomes a dipole because of the interaction with an ion or polar molecule
results when an ion or a dipole induces a temporary dipole in an atom or a molecule near it
a bond created when a highly electronegative atom on one molecule attracts the positively charged hydrogen atom on a nearby molecule
