Chemical Change: Identify and apply types of chemical reactions
Unit 1: Types of chemical reactions
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- identify the following types of chemical reactions:
- Combination
- Decomposition
- Single displacement.
What you should know
Before you start this unit, make sure you can:
Balance chemical equations. Refer to level 3 subject outcome 6.2 unit 1 to revise this.
Introduction
Parts of the text in this unit were sourced from chem.libretexts.org, Chapter: Descriptive Chemistry, released under a CC-BY-NC-SA 3.0 licence.
Chemical reactions are the processes by which chemicals interact to form new chemicals with different compositions. Simply stated, a chemical reaction is the process where reactants are transformed into products. How chemicals react is dictated by the chemical properties of the element or compound – the ways in which a compound or element undergoes changes in composition.
Combination reactions
A is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions. The general form of a combination reaction is shown in figure 1.

Here are some examples of simple combination reactions when two elements combine to form a compound:
- Solid sodium metal reacts with chlorine gas to produce solid sodium chloride
[latex]\scriptsize \displaystyle \text{2N}{{\text{a}}_{{\left( \text{s} \right)}}}\text{+C}{{\text{l}}_{{\text{2}\left( \text{g} \right)}}}\to 2\text{NaC}{{\text{l}}_{{\left( \text{s} \right)}}}[/latex] - Sulfur reacts with oxygen to form sulfur dioxide
[latex]\scriptsize \displaystyle {{\text{S}}_{{\left( \text{s} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{S}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex] - Often, nonmetal reactants can combine in different ratios and produce different products. Sulfur can also combine with oxygen to form sulfur trioxide.
[latex]\scriptsize \displaystyle \text{2}{{\text{S}}_{{\left( \text{s} \right)}}}\text{+3}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2S}{{\text{O}}_{{\text{3}\left( \text{g} \right)}}}[/latex] - Sulfur trioxide gas reacts with water to form sulfuric acid. This is, unfortunately, a common reaction that occurs in the atmosphere in some places where oxides of sulfur are present as pollutants. The acid formed in the reaction falls to the ground as acid rain:
[latex]\scriptsize \displaystyle \text{S}{{\text{O}}_{{\text{3}\left( \text{g} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to {{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}_{{\left( {\text{aq}} \right)}}[/latex]
Combination reactions can also take place when an element reacts with a compound to form a new compound composed of a larger number of atoms. Carbon monoxide reacts with oxygen to form carbon dioxide according to the equation:
[latex]\scriptsize \displaystyle \text{2C}{{\text{O}}_{{\left( \text{g} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Two compounds may also react to form a more complex compound. A common example is the reaction of an oxide with water. Calcium oxide reacts readily with water to produce an aqueous solution of calcium hydroxide:
[latex]\scriptsize \displaystyle \text{Ca}{{\text{O}}_{{\left( \text{s} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{Ca}{{\left( {\text{OH}} \right)}_{{2\left( {aq} \right)}}}[/latex]
Reactions with oxygen
One sort of combination reaction that occurs frequently is the reaction of an element with oxygen to form an oxide. Metals and nonmetals both react readily with oxygen under most conditions. Oxides of metals are , and oxides of nonmetals are .
Group 1 alkali metals
Oxygen reacts rapidly with group 1 elements. All form solutions when dissolved in water. The oxide [latex]\scriptsize \displaystyle {{\text{M}}_{\text{2}}}\text{O}[/latex] (where M represents any alkali metal) can be formed with any of the alkali metals. When heated, lithium, sodium, potassium, rubidium, and caesium ignite through combustion reactions with oxygen.
Lithium reacts with oxygen to form [latex]\scriptsize \displaystyle \text{Li}{{}_{\text{2}}}\text{O}[/latex]and burns with a red flame.
[latex]\scriptsize \displaystyle \text{4L}{{\text{i}}_{{\left( \text{s} \right)}}}\text{+ }{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2L}{{\text{i}}_{\text{2}}}{{\text{O}}_{{\left( \text{s} \right)}}}[/latex]
Sodium burns in air with often little more than an orange glow. Using larger amounts of sodium or burning it in pure oxygen produces a strong orange flame.
[latex]\scriptsize \displaystyle \text{4N}{{\text{a}}_{{\left( \text{s} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2N}{{\text{a}}_{\text{2}}}{{\text{O}}_{{\left( \text{s} \right)}}}[/latex]

Pieces of potassium burn with a lilac-coloured flame.
[latex]\scriptsize \displaystyle \text{4}{{\text{K}}_{{\left( \text{s} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to 2{{\text{K}}_{\text{2}}}{{\text{O}}_{{\left( \text{s} \right)}}}[/latex]
Group 2 alkali earth metals
The elements of group 2 also react with oxygen, though not as rapidly as group 1 metals; these reactions also require heating. Similarly to group 1 oxides, most group 2 oxides are only slightly soluble in water and form basic solutions. All group 2 metals react similarly, burning to form oxides as shown:
[latex]\scriptsize \displaystyle \text{2}{{\text{M}}_{{\left( \text{s} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2M}{{\text{O}}_{{\left( \text{s} \right)}}}[/latex]
Once started, the reactions with oxygen are vigorous except beryllium which does not react with air or water.
Magnesium reacts rapidly and dramatically when ignited, combining with oxygen from the air to produce a fine powder of magnesium oxide:
[latex]\scriptsize \displaystyle \text{2M}{{\text{g}}_{{\left( \text{s} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2Mg}{{\text{O}}_{{\left( \text{s} \right)}}}[/latex]

Group 13 elements
Group 13 elements vary in their reactions with oxygen. All group 13 elements also produce compounds of the form of [latex]\scriptsize \displaystyle \text{M}{{}_{\text{2}}}{{\text{O}}_{\text{3}}}[/latex]. Here is the equation of the reaction of oxygen and a group 13 element:
[latex]\scriptsize \displaystyle \text{4}{{\text{M}}_{{\left( \text{s} \right)}}}\text{+3}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2}{{\text{M}}_{\text{2}}}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}[/latex]
The most common oxide form of boron, [latex]\scriptsize \displaystyle {{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}}[/latex] or boron trioxide, is obtained by heating boric acid.
Aluminium occurs almost exclusively in the [latex]\scriptsize \displaystyle +\text{ }3[/latex] oxidation state. It rapidly reacts with oxygen in air to give a water-insoluble coating of [latex]\scriptsize \displaystyle \text{A}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}[/latex]. This oxide layer protects the metal beneath from further corrosion. The reaction is shown below:
[latex]\scriptsize \displaystyle \text{4A}{{\text{l}}_{{\left( \text{s} \right)}}}\text{+3}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2A}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}[/latex]
Transition metals
Transition metals can adopt multiple positive charges within their ionic compounds. Therefore, most transition metals can form different products in a combination reaction.
Iron reacts with oxygen to form both iron (II) oxide and iron (III) oxide which is otherwise known as rust:
[latex]\scriptsize \displaystyle \begin{align*}& {\text{2F}{{\text{e}}_{{\left( \text{s} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2Fe}{{\text{O}}_{{\left( \text{s} \right)}}}} \\& {\text{4F}{{\text{e}}_{{\left( \text{s} \right)}}}\text{+3}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2F}{{\text{e}}_{\text{2}}}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}} \end{align*}[/latex]
Metalloid and nonmetal reactions with oxygen
Group 14 elements
Group 14 is made up of both metals (towards the bottom of the group), metalloids, and nonmetals (at the top of the group). The oxides towards the top of the group 14 elements are slightly acidic, and the acidity of the oxides decreases down the group.
- Non-metal: The non-metal carbon burns to form [latex]\scriptsize \displaystyle \text{C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex] and, when oxygen is present in smaller amounts, [latex]\scriptsize \displaystyle \text{C}{{\text{O}}_{{\left( \text{g} \right)}}}[/latex]; both are acidic under different conditions. Carbon monoxide is only slightly soluble in water and does not react with it.
- Metalloid: The metalloid silicon reacts with oxygen to form only one stable compound, [latex]\scriptsize \displaystyle \text{SiO}{{}_{\text{2}}}[/latex].
- The three metals in this group have many different oxide compounds because they do not obey the octet rule. For example:
Germanium: [latex]\scriptsize \displaystyle \text{GeO, }\!\!~\!\!\text{ Ge}{{\text{O}}_{\text{2}}}[/latex]
Tin: [latex]\scriptsize \displaystyle \text{SnO, }\!\!~\!\!\text{ SnO}{{}_{\text{2}}}[/latex]
Lead: [latex]\scriptsize \displaystyle \text{PbO, }\!\!~\!\!\text{ Pb}{{\text{O}}_{\text{2}}}\text{, }\!\!~\!\!\text{ P}{{\text{b}}_{\text{3}}}{{\text{O}}_{\text{4}}}[/latex]
Group 15 elements
The nitrogen family, Group 15, can react with oxygen in many ways. Nitrogen and phosphorus are non-metallic, arsenic and antimony are metalloids, and bismuth is metallic.
Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from [latex]\scriptsize +1[/latex] to [latex]\scriptsize +5[/latex]: All these oxides are gases at room temperature except for [latex]\scriptsize \displaystyle \text{N}{{}_{\text{2}}}{{\text{O}}_{\text{5}}}[/latex], which is solid. The nitrogen oxides are: [latex]\scriptsize \displaystyle \text{NO, }{{\text{N}}_{\text{2}}}\text{O, }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{, N}{{\text{O}}_{\text{2}}}\text{, }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}[/latex].
All these reactions are endothermic, requiring energy for oxygen to react directly with [latex]\scriptsize \displaystyle {{\text{N}}_{{\text{2(g)}}}}[/latex] and the products are acidic.
Phosphorus reacts with oxygen, usually forming two oxides depending on the amount of available oxygen: it forms [latex]\scriptsize \displaystyle ~{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}[/latex] when reacted with a limited supply of oxygen, and [latex]\scriptsize \displaystyle {{\text{P}}_{{\text{4}}}}{{\text{O}}_{{\text{10}}}}[/latex] when reacted with excess oxygen; the latter is shown below.
[latex]\scriptsize \displaystyle {{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\text{+6}{{\text{H}}_{\text{2}}}\text{O}\to \text{4}{{\text{H}}_{\text{3}}}\text{PO}[/latex]
Group 16 elements
The elements in group 16 include oxygen, sulfur, selenium, tellurium, and polonium. Oxygen reacts with the elements in its own group to form various oxides, mostly in the form of [latex]\scriptsize \displaystyle \text{A}{{\text{O}}_{\text{2}}}[/latex] and [latex]\scriptsize \displaystyle \text{A}{{\text{O}}_{\text{3}}}[/latex] where A represents a group 16 element.
Although oxygen is in group 16, it is unique in its extreme electronegativity; this allows it to readily gain electrons. Because it is the smallest element in its group, it can form double bonds.
Oxygen can react with itself, forming : ozone ([latex]\scriptsize \displaystyle {{\text{O}}_{\text{3}}}[/latex]), and dioxygen ([latex]\scriptsize {{\text{O}}_{\text{2}}}[/latex]).
Sulfur’s reaction with oxygen produces the oxides mentioned above. Sulfur dioxide, [latex]\scriptsize \displaystyle \text{S}{{\text{O}}_{2}}[/latex], and sulfur trioxide, [latex]\scriptsize \displaystyle \text{S}{{\text{O}}_{3}}[/latex], are the only common sulfur oxides.
[latex]\scriptsize \displaystyle {{\text{S}}_{{\left( \text{s} \right)}}}\text{+ }{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{S}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Selenium and tellurium adopt compounds of the forms [latex]\scriptsize \displaystyle \text{A}{{\text{O}}_{\text{2}}}\text{, A}{{\text{O}}_{\text{3}}}[/latex], and [latex]\scriptsize \displaystyle \text{AO}[/latex].
Group 17 elements: The halogens
The halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. They range in structure from [latex]\scriptsize \displaystyle {{\text{X}}_{{\text{2}}}}\text{O}[/latex] to [latex]\scriptsize \displaystyle \text{X}{{}_{\text{2}}}{{\text{O}}_{\text{7}}}[/latex], where X represents a halogen. Their extended octets allow them to bond with many oxygen atoms at a time.
The most electronegative element adopts the [latex]\scriptsize \displaystyle -1[/latex] oxidation state. Fluorine and oxygen form [latex]\scriptsize \displaystyle \text{O}{{\text{F}}_{2}}[/latex], which is known as oxygen fluoride.
[latex]\scriptsize \displaystyle \text{2}{{\text{F}}_{\text{2}}}\text{ + 2NaOH}\to \text{O}{{\text{F}}_{\text{2}}}\text{ + 2NaF + }{{\text{H}}_{\text{2}}}\text{O}[/latex]
Group 18 elements: The noble gases
Noble gases are chemically inert except for xenon, which reacts with oxygen to form [latex]\scriptsize \displaystyle \text{Xe}{{\text{O}}_{{\text{3}}}}[/latex] and [latex]\scriptsize \displaystyle \text{XeO}{{}_{\text{4}}}[/latex] at low temperatures and high pressures. [latex]\scriptsize \displaystyle \text{Xe}{{\text{O}}_{{\text{3}}}}[/latex] is highly unstable, and is known to spontaneously detonate in a clean, dry environment.
Exercise 1.1
- If there are [latex]\scriptsize \displaystyle 4.00\text{ g}[/latex] of potassium, how much [latex]\scriptsize \displaystyle {{\text{K}}_{\text{2}}}\text{O}[/latex] is created during combustion (in excess oxygen)?
- Which noble gas(es), if any, react with oxygen?
- Complete and balance the following reaction: [latex]\scriptsize \displaystyle \text{A}{{\text{l}}_{{\left( \text{s} \right)\text{ }}}}\text{+ }{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to[/latex]
- What is the best environment for nitrogen and oxygen to react in?
- What are the principal combustion products of each group 1 element with oxygen?
The full solutions can be found at the end of the unit.
Oxidation and reduction
is the loss of electrons from a substance. It is also the gain of oxygen by a substance. For example, magnesium is oxidised when it reacts with oxygen to form magnesium oxide:
[latex]\scriptsize \displaystyle \text{2Mg + }{{\text{O}}_{\text{2}}}\text{ }\to \text{2MgO}[/latex]
is the gain of electrons by a substance. It is also the loss of oxygen from a substance. For example, copper(II) oxide can be reduced to form copper when it reacts with hydrogen:
[latex]\scriptsize \displaystyle \text{CuO + }{{\text{H}}_{\text{2}}}\text{ }\to \text{Cu + }{{\text{H}}_{\text{2}}}\text{O}[/latex]
Usually, oxidation and reduction take place at the same time in a reaction. We call this type of reaction a .
For example when aluminium reacts with iron oxide:
aluminium + iron(III) oxide → iron + aluminium oxide
It is easy to see that the aluminium has been oxidised. This means that the iron oxide is the oxidising agent. We can also see that the iron oxide has been reduced. This means that the aluminium is the reducing agent.
Rusting is an oxidation reaction. The iron reacts with water and oxygen to form hydrated iron(III) oxide, which we see as rust.
Take note!
The oxidising agent is the chemical that causes oxidation.
The reducing agent causes the other chemical to be reduced.
Single displacement reactions
A involves a metal and a compound of a different metal. In a displacement reaction a more reactive metal will displace a less reactive metal from its compound.
Single displacement reactions occur between metals because the ions of low-ranking metals are readily reduced by high-ranking metals on the reactivity series. Therefore, low ranking metals are easily displaced by high-ranking metals in the single displacement reactions between them.
This concept has several practical applications in the extraction of metals. For example, titanium is extracted from titanium tetrachloride via a single displacement reaction with magnesium.
The reactivity series of metals can also be used to predict the outcome of single displacement reactions. A displacement reaction can also be a combination reaction.
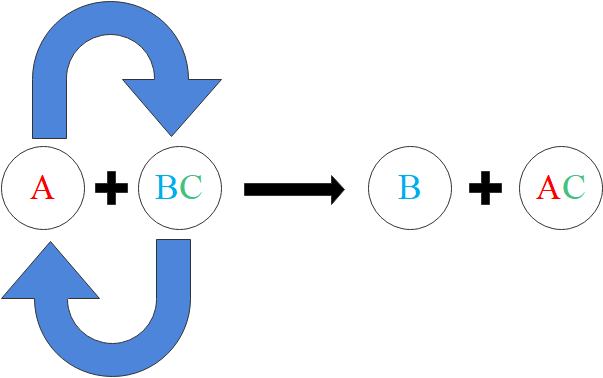
Displacement reactions are easily seen when a more reactive metal is placed in a solution of a salt of the less reactive metal. During the reaction, the more reactive metal gradually disappears as it forms a solution and the less reactive metal ions precipitate out as the solid metal. For example, magnesium is more reactive than copper:
[latex]\scriptsize \text{M}{{\text{g}}_{{\text{(s)}}}}\text{+CuS}{{\text{O}}_{\text{4}}}_{{\text{(aq)}}}\to \text{MgS}{{\text{O}}_{\text{4}}}_{{\text{(aq)}}}\text{+ C}{{\text{u}}_{{\text{(s)}}}}[/latex]
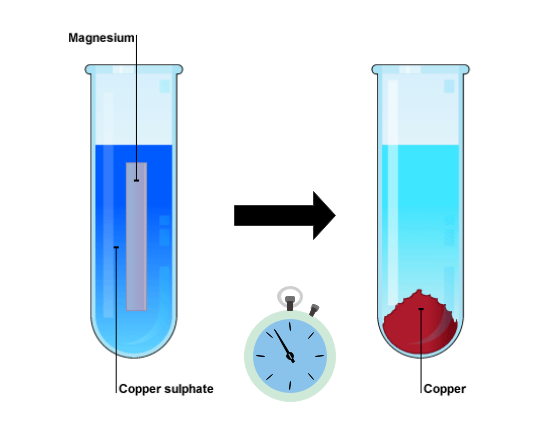
No reaction is seen if you do things the other way round – in other words if you put copper powder into magnesium sulfate solution. This is because copper is not reactive enough to displace magnesium from magnesium sulfate.
Further examples of single displacement reactions:
Hydrogen in hydrogen chloride is replaced by zinc, and in the process hydrogen and zinc chloride are formed.
[latex]\scriptsize \displaystyle \text{2HC}{{\text{l}}_{{\left( {\text{aq}} \right)}}}\text{+Z}{{\text{n}}_{{\left( \text{s} \right)}}}\to \text{ZnC}{{\text{l}}_{{\text{2}\left( {\text{aq}} \right)}}}\text{+}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Another example of a single-replacement reaction is:
[latex]\scriptsize \displaystyle \text{2NaC}{{\text{l}}_{{\left( {\text{aq}} \right)}}}\text{+}{{\text{F}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2Na}{{\text{F}}_{{\left( \text{s} \right)}}}\text{+C}{{\text{l}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
A typical characteristic of a single-replacement reaction is that there is one element as a reactant and another element as a product.
Displacement reactions of metal oxides
A more reactive metal will displace a less reactive metal from a compound. The is a good example of this. A thermite reaction is an exothermic reaction between ferrous oxides and aluminium. It is used to produce white hot molten (liquid) iron in remote locations for welding. A lot of heat is needed to start the reaction, but then it releases an incredible amount of heat, enough to melt the iron.
[latex]\scriptsize \displaystyle \text{2Al + F}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{ }\to \text{ 2Fe + A}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}[/latex]
Because aluminium is more reactive than iron, it displaces iron from iron(III) oxide. The aluminium removes oxygen from the iron(III) oxide: iron is reduced, and the aluminium is oxidised.
Note
To see more about this, you can watch the thermite reaction, by WTN Chemistry.
Reactions between metals and metal oxides allow us to put a selection of metals into a reactivity series. In general, the greater the difference in reactivity between two metals in a displacement reaction, the greater the amount of energy released.
Aluminium is much higher than iron in the reactivity series, so the thermite reaction releases a lot of energy. Magnesium is very high in the reactivity series, and copper is very low – so the reaction between magnesium and copper oxide is more violent.
The reactivity series
The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in descending order of their reactivities.
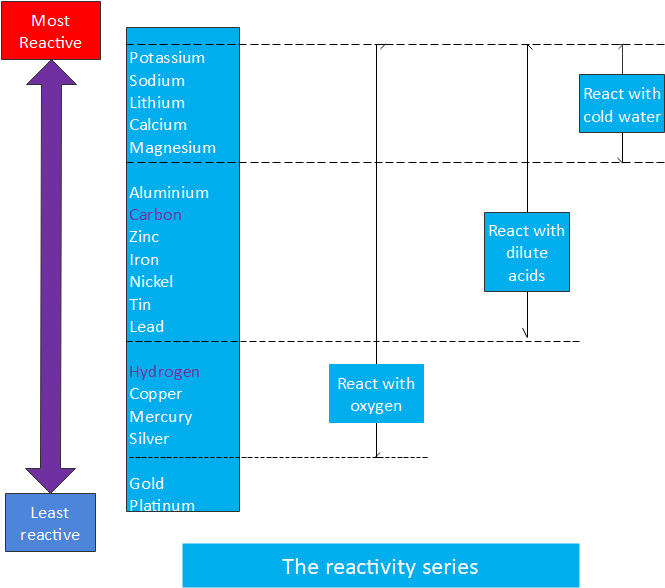
Metals tend to readily lose electrons and form cations. Most of them react with atmospheric oxygen to form metal oxides. However, different metals have different levels of reactivity with oxygen. The unreactive metals such as gold and platinum do not readily form oxides when exposed to air.
Important points:
- All metals that are found above hydrogen in the reactivity series liberate [latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}[/latex] gas upon reacting with dilute hydrochloric acid ([latex]\scriptsize \displaystyle \text{HCl}[/latex]) or dilute sulfuric acid ([latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}[/latex]).
- Metals that are placed higher on the reactivity series have the ability to displace metals that are placed lower, from their salt solutions.
- Higher ranking metals require greater amounts of energy for their isolation from ores and other compounds.
- Another important feature of the reactivity series is that while moving down the series, the electron-donating ability of the metals decreases.
Apart from providing insight into the properties and reactivities of the metals, the reactivity series has several other important applications. For example, the outcome of the reactions between metals and water, metals and acids, and single displacement reactions between metals can be predicted with the help of the reactivity series.
Remember: non-metals get more reactive as you move up the group in the periodic table. This is particularly true of the halogens, where bromine is more reactive that iodine but less reactive than chlorine.
Exercise 1.2
- Which is the most reactive of these three metals?
- Lithium
- Copper
- Zinc
- Which of the following combinations will result in a displacement reaction?
- Copper and zinc nitrate
- Zinc and magnesium nitrate
- Magnesium and copper sulfate
- What has been reduced in this reaction: iron oxide + carbon → iron + carbon monoxide?
- Iron oxide
- Carbon
- Carbon monoxide
- What is the oxidising agent in this reaction: lead oxide + carbon → lead + carbon monoxide?
- Lead oxide
- Carbon
- Lead
The full solutions can be found at the end of the unit.
Reaction between elements and water
Water is composed of two hydrogen atoms and an oxygen atom. It exhibits polarity and is naturally found in the liquid, solid, and vapour states. Its polarity makes it a good solvent and it is commonly known as the universal solvent. Because of its abundance on Earth, it is important to note that it is involved in many chemical reactions. Many of these chemical reactions behave in trends that can be categorised using the periodic table.
Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding hydroxide while giving off hydrogen gas. For example, the reaction between potassium and water yields potassium hydroxide and [latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}[/latex] gas, as described by the chemical equation: [latex]\scriptsize \displaystyle \text{2}{{\text{K}}_{{\text{(s)}}}}\text{+ 2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\text{(l)}}}}\text{ }\to \text{ 2KO}{{\text{H}}_{{\text{(aq)}}}}\text{ + }{{\text{H}}_{\text{2}}}_{{(g)}}[/latex]
Therefore, the reactivity series of metals can be used to predict the reactions between metals and water.
Group 1: Alkali metals
A common characteristic of most alkali metals is their ability to displace [latex]\scriptsize \displaystyle {{\text{H}}_{{\text{2}\left( \text{g} \right)}}}[/latex] from water. In this event, the group 1 metal is to its metal ion and water is to form hydrogen gas and hydroxide ions. The general reaction of an alkali metal (M) with H2O (l) is given in the following equation:
[latex]\scriptsize \displaystyle \text{2}{{\text{M}}_{{\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{2}{{\text{M}}^{\text{+}}}_{{\left( {\text{aq}} \right)}}\text{+2O}{{\text{H}}^{\text{-}}}_{{\left( {\text{aq}} \right)}}\text{+}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Group 1 elements are called alkali metals because of their ability to displace [latex]\scriptsize \displaystyle {{\text{H}}_{{\text{2}\left( \text{g} \right)}}}[/latex] from water and create a basic solution.
Alkali metals are also known to react violently and explosively with water. This is because enough heat is given off during the exothermic reaction to ignite the [latex]\scriptsize \displaystyle {{\text{H}}_{{\text{2}\left( \text{g} \right)}}}[/latex].



Group 1: Alkali metals oxides and water
Oxides of group 1 elements also react with water to create basic solutions. Alkali metals react with oxygen to form monoxides, peroxides, or superoxides. These species react with water in different ways:
Monoxides ([latex]\scriptsize \displaystyle {{\text{M}}_{\text{2}}}\text{O}[/latex]) produce alkali metal hydroxides: [latex]\scriptsize \displaystyle {{\text{M}}_{\text{2}}}{{\text{O}}_{{\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{2}{{\text{M}}^{\text{+}}}_{{\left( {\text{aq}} \right)}}\text{+2O}{{\text{H}}^{\text{-}}}_{{\left( {\text{aq}} \right)}}[/latex]
Peroxides ([latex]\scriptsize \displaystyle \text{M}{{}_{\text{2}}}{{\text{O}}_{\text{2}}}[/latex]) produce metal hydroxides and hydrogen peroxide:[latex]\scriptsize \displaystyle {{\text{M}}_{\text{2}}}{{\text{O}}_{{\text{2}\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{2}{{\text{M}}^{\text{+}}}_{{\left( {\text{aq}} \right)}}\text{+2O}{{\text{H}}^{\text{-}}}_{{\left( {\text{aq}} \right)}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\text{2}\left( {\text{aq}} \right)}}}[/latex]
Superoxides ([latex]\scriptsize \displaystyle \text{M}{{\text{O}}_{\text{2}}}[/latex]) produce metal hydroxides, hydrogen peroxide, and oxygen gas:
[latex]\scriptsize \displaystyle \text{2M}{{\text{O}}_{{\text{2}\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{2}{{\text{M}}^{\text{+}}}_{{\left( {\text{aq}} \right)}}\text{+2O}{{\text{H}}^{\text{-}}}_{{\left( {\text{aq}} \right)}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\text{2}\left( {\text{aq}} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Alkali metal hydrides and water
Similarly to the group 1 oxides, the of the group 1 elements react with water to form a basic solution. In this case, however, hydrogen gas is produced with the metal hydroxide. The general reaction for alkali metal hydrides and water is given below:
[latex]\scriptsize \displaystyle \text{M}{{\text{H}}_{{\left( \text{s} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to {{\text{M}}^{\text{+}}}_{{\left( {\text{aq}} \right)}}\text{+O}{{\text{H}}^{\text{-}}}_{{\left( {\text{aq}} \right)}}\text{+}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
This reaction can be generalized to all alkali metal hydrides.
Group 2: Alkaline earth metals
Most alkaline earth metals also produce hydroxides when reacted with water. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; however, enough hydroxide ions are produced to make a basic solution. The general reaction of calcium, strontium, and barium with water is represented below, where M represents calcium, strontium, or barium:
[latex]\scriptsize \displaystyle {{\text{M}}_{{\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{M}{{\left( {\text{OH}} \right)}_{{\text{2}\left( {\text{aq}} \right)}}}\text{+}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Magnesium does not react with water but reacts with steam to form magnesium hydroxide and hydrogen gas:
[latex]\scriptsize \displaystyle \text{M}{{\text{g}}_{{\left( \text{s} \right)\text{ }}}}\text{+ }{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}\to \text{ Mg}{{\text{O}}_{{\left( \text{s} \right)}}}\text{ + }{{\text{H}}_{\text{2}}}_{{\left( \text{g} \right)}}[/latex]
Alkaline earth metal oxides and water
Similarly to the alkali metal oxides, alkaline earth metal monoxides combine with water to form metal hydroxide salts except for beryllium, whose oxide ([latex]\scriptsize \displaystyle \text{BeO}[/latex]) does not react with water.
The general equation for these reactions are: [latex]\scriptsize \displaystyle \text{M}{{\text{O}}_{{\left( \text{s} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{M}{{\left( {\text{OH}} \right)}_{{\text{2}\left( \text{s} \right)}}}[/latex]
One of the most familiar alkaline earth metal oxides is [latex]\scriptsize \displaystyle \text{CaO}[/latex] or quicklime. This substance is often used to treat water and to remove harmful [latex]\scriptsize \displaystyle \text{S}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex] from industrial smokestacks.
Alkaline earth metal hydrides and water
Except for beryllium, the alkaline metal hydrides react with water to produce the metal hydroxide and hydrogen gas. The reaction of these metal hydrides can be described:[latex]\scriptsize \displaystyle \text{M}{{\text{H}}_{{\text{2}\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{M}{{\left( {\text{OH}} \right)}_{{\text{2}\left( {\text{aq}} \right)}}}\text{+2}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Nonmetal and metalloid groups
Nonmetal and metalloid groups, groups 13 to 18, are generally not very reactive to water.
- Group 13 elements are not very reactive with water and boron does not react with water. Aluminium does not react with water because an outer layer of aluminium oxide ([latex]\scriptsize \displaystyle \text{A}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}[/latex]) solid forms and protects the rest of the metal.
- For the most part, group 14 elements do not react with water. One interesting consequence of this is that tin (Sn) is often sprayed as a protective layer on iron cans to prevent the can from corroding.
- the elements in group 15 do not tend to react with water. Compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas dissolve in water but do not react.
- Oxygen is found in group 16 and as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. The nonmetal oxides react with water to form oxoacids. Examples include phosphoric acid and sulfuric acid.
- Generally group 17 halogens react with water to give . Because fluorine is so electronegative, it can displace oxygen gas from water. The products of this reaction include oxygen gas and hydrogen fluoride.
[latex]\scriptsize \displaystyle \text{2}{{\text{F}}_{{\text{2(g)}}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\text{(l)}}}}\to \text{4H}{{\text{F}}_{{\text{(aq)}}}}\text{+}{{\text{O}}_{{\text{2(g)}}}}[/latex]
The hydrogen halides react with water to form acids and, except for HF, they are strong acids in water. Hydrochloric acid ([latex]\scriptsize \displaystyle \text{HCl}[/latex]) is a strong acid, for example. - Group 18, the noble gases, do not react with water.
Exercise 1.3
- Predict the products of the following reactions:
- [latex]\scriptsize \displaystyle \text{B}{{\text{e}}_{{\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to[/latex]
- [latex]\scriptsize \displaystyle \text{N}{{\text{e}}_{{\left( \text{g} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to[/latex]
- [latex]\scriptsize \displaystyle \text{L}{{\text{i}}_{\text{2}}}{{\text{O}}_{{\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to[/latex]
- True/False
- Metal oxides form basic solutions in water.
- Sodium is the alkali element that reacts most violently with water.
- Does aluminium react with water? Explain your answer.
The full solutions can be found at the end of the unit.
Reaction between metals and acids
Metals react with acids at different rates, depending on how reactive the metals are. Hydrogen can be produced from acids when they react with metals. Lead and the metals ranking above lead on the reactivity series form salts when reacted with hydrochloric acid or sulphuric acid.
These dilute acids react with relatively reactive metals such as magnesium, aluminium, zinc, and iron. In general, the more reactive the metal, the more quickly and/or violent the reaction.
| Metal | Reaction with hydrochloric acid |
| Potassium | Reacts explosively |
| Sodium | Reacts explosively |
| Calcium | Reacts violently |
| Magnesium | Reacts rapidly |
| Aluminium | Has a protective oxide layer, so it reacts slowly with acids to begin with but then reacts rapidly |
| Zinc | Reacts moderately |
| Iron | Reacts slowly |
The products of these reactions are a salt plus hydrogen gas.
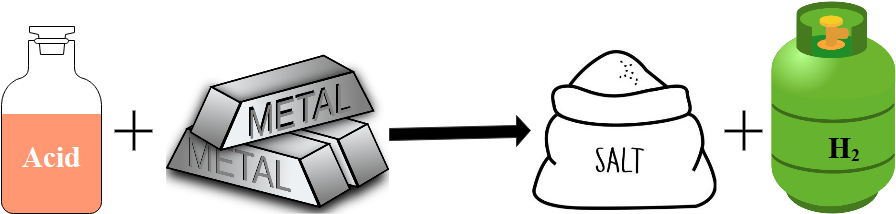
The name of the salt depends on the name of the acid. For example, when magnesium reacts with hydrochloric acid magnesium chloride and hydrogen gas are produced:
[latex]\scriptsize \displaystyle \text{M}{{\text{g}}_{{\text{(s)}}}}\text{+ 2HC}{{\text{l}}_{{\text{(aq)}}}}\to \text{MgC}{{\text{l}}_{{\text{2 }}}}_{{\text{(aq)}}}\text{+ }{{\text{H}}_{\text{2}}}_{{\text{(g)}}}[/latex]
The reaction between zinc and sulfuric acid forms zinc sulfate and hydrogen gas:
[latex]\scriptsize \displaystyle \text{Z}{{\text{n}}_{{\text{(s)}}}}\text{ + }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}_{{\text{(aq)}}}\text{ }\to \text{ZnS}{{\text{O}}_{\text{4}}}_{{\text{(aq)}}}\text{ + }{{\text{H}}_{\text{2}}}_{{\text{(g)}}}[/latex]
When magnesium reacts with a dilute solution of nitric acid, magnesium nitrate and hydrogen are produced:
[latex]\scriptsize \displaystyle \text{M}{{\text{g}}_{{\text{(s)}}}}\text{ + 2HN}{{\text{O}}_{\text{3}}}_{{\text{(aq)}}}\text{ }\to \text{Mg}{{\left( {\text{N}{{\text{O}}_{\text{3}}}} \right)}_{{\text{2(aq)}}}}\text{ + }{{\text{H}}_{{\text{2(g)}}}}[/latex]
The salt produced depends on the type of acid involved in the reaction:
- when a metal reacts with hydrochloric acid, the salt produced is a metal chloride
- when a metal reacts with sulfuric acid, the salt produced is a metal sulfate
- when a metal reacts with nitric acid, the salt produced is a metal nitrate.
Displacement of halogens and halides
Generally, halogens are good electron acceptors and therefore are good oxidising agents. When going down group 17, the size of the halogen atoms increases. The nucleus is further away from the outermost occupied shell. So the electronegativity of halogens or their ability to accept electrons to form negatively charged halide ions decreases down the group. As a result, the strength of halogens as oxidising agents decreases down the group.
In a displacement of a halogen, a more electronegative halogen displaces a less electronegative halogen from its halide solution. Halogens oxidise halide ions. The halide ion must be lower down in group 17. For example in the reaction between sodium bromide and chlorine, the bromide will be displaced by chlorine, because chlorine is more reactive than bromine.
[latex]\scriptsize \text{2NaB}{{\text{r}}_{{\text{(aq)}}}}\text{+ C}{{\text{l}}_{{\text{2(g)}}}}\to \text{NaC}{{\text{l}}_{{\text{(aq)}}}}\text{+B}{{\text{r}}_{{\text{2(l)}}}}[/latex]
The halide ions of the less electronegative halogen act as the reducing agent. They lose their electrons and are oxidised to form halogen molecules. The electrons are accepted by the more electronegative halogen which acts as the oxidising agent. By doing so, the halogen undergoes reduction to form its halide ion.
Chlorine is more reactive than iodine. A solution of chlorine can displace iodine from potassium iodide solution:
chlorine + potassium iodide → potassium chloride + iodine
[latex]\scriptsize \displaystyle \text{C}{{\text{l}}_{{\text{2}\left( {\text{aq}} \right)}}}\text{ + 2K}{{\text{I}}_{{\left( {\text{aq}} \right)}}}\to \text{ 2KC}{{\text{l}}_{{\left( {\text{aq}} \right)}}}\text{ + }{{\text{I}}_{{\text{2}\left( {\text{aq}} \right)}}}[/latex]
Note
You can watch the video Displacement of Halogens, by The Rugby School.
Decomposition reactions
are common and occur when one chemical splits into two or more chemicals, like this:
Mercury (II) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas:
[latex]\scriptsize \displaystyle \text{2Hg}{{\text{O}}_{{\left( \text{s} \right)}}}\to \text{2H}{{\text{g}}_{{\left( \text{l} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Hydrogen peroxide will decompose when it is exposed to light, high temperatures, and when diluted in water to form water and oxygen:
[latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}_{{\text{(aq)}}}\rightleftharpoons {{\text{H}}_{\text{2}}}{{\text{O}}_{{\text{(l)}}}}\text{+}{{\text{O}}_{{\text{2(g)}}}}[/latex]
Large amounts of aluminium are produced from the decomposition of aluminium oxide. This is done by applying heat and electricity to solutions of aluminium oxide:
[latex]\scriptsize \text{2A}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}_{{\text{(s)}}}\to \text{4A}{{\text{l}}_{{\text{(s)}}}}\text{+3}{{\text{O}}_{{\text{2(g)}}}}[/latex]
Decomposition reactions happen all around us and are often a result from heating a substance. For instance, when heated, a salt may lose a gas like oxygen or carbon dioxide, leaving behind a simpler salt or metal. This could happen explosively, depending on the compound. Or, when heated, a metal hydroxide loses water to form the metal oxide. For example, ammonium dichromate decomposes on heating to produce nitrogen gas, water vapour, and solid chromium (III) oxide:
[latex]\scriptsize \displaystyle {{\left( {\text{N}{{\text{H}}_{\text{4}}}} \right)}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{{\text{7}\left( \text{s} \right)}}}\text{ }\to \text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}\text{ + }{{\text{N}}_{\text{2}}}_{{\text{(g)}}}\text{ + 4 }{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}[/latex]
While the ammonium dichromate decomposes, it gives off orange sparks and the green chromium (III) oxide crystals are projected into the air, producing an effect that looks like a miniature volcanic eruption.
Note
You can watch a demonstration of this reaction by Mr Lund Science called Volcano Reaction – Ammonium Dichromate Decomposition.
A decomposition reaction can be both endothermic or exothermic. However, exothermic reactions are more common than endothermic ones. For example, the decomposition of [latex]\scriptsize \displaystyle \text{NO}[/latex] to [latex]\scriptsize \displaystyle {{\text{N}}_{\text{2}}}[/latex] and [latex]\scriptsize \displaystyle {{\text{O}}_{\text{2}}}[/latex] is exothermic, so is the decomposition of ozone to oxygen.
Most decomposition reactions require an input of energy in the form of heat, light, or electricity.
Decomposition reactions can be classified into three types:
- thermal decomposition
- electrolytic decomposition
- photo decomposition.
Thermal decomposition
In thermal decomposition, a single substance breaks into two or more substances when heated. Since heat is required to break the bonds, these reactions are endothermic. The following compounds undergo thermal decomposition.
- Carbonates: Carbonates are compounds formed between a metal and the carbonate ion, [latex]\scriptsize \displaystyle {{\left( {\text{C}{{\text{O}}_{\text{3}}}} \right)}^{{\text{2-}}}}[/latex]. When a carbonate decomposes, a metal oxide and carbon dioxide gas are produced.
.
Calcium carbonate (limestone) decomposes into calcium oxide or quick lime, which is the main ingredient of cement, and carbon dioxide:
[latex]\scriptsize \displaystyle \text{CaC}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}\to \text{Ca}{{\text{O}}_{{\left( \text{s} \right)}}}\text{+C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
.
Sodium carbonate decomposes to form sodium oxide and carbon dioxide gas:
[latex]\scriptsize \displaystyle \text{N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}\to \text{N}{{\text{a}}_{\text{2}}}{{\text{O}}_{{\left( \text{s} \right)}}}\text{ + C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex] - Chlorates are compounds formed between a metal and the chlorate ion,[latex]\scriptsize \displaystyle {{\left( {\text{Cl}{{\text{O}}_{\text{3}}}} \right)}^{{\text{1-}}}}[/latex]. When a chlorate decomposes, a metal chloride and oxygen gas are produced. Chlorates are often referred to as oxidisers because they produce their own oxygen when they react.
.
Potassium chlorate decomposes into potassium chloride and oxygen when it is strongly heated:
[latex]\scriptsize \displaystyle \text{2KCl}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}\to \text{2KC}{{\text{l}}_{{\left( \text{s} \right)}}}\text{+3}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex] - Nitrates: Nitrates of highly reactive metals decompose thermally to form a metal nitrite and oxygen gas when heated. Nitrates of moderately reactive metals produce toxic brown fumes of nitrogen dioxide gas when heated, as well as the metal oxide and oxygen gas. Nitrates of low reactive metals decompose thermally to form the metal, nitrogen dioxide and oxygen.
.
Lead nitrate will decompose into lead oxide, nitrogen dioxide and oxygen:
[latex]\scriptsize \text{2Pb(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}_{{\text{(s)}}}\to \text{2Pb}{{\text{O}}_{{\text{(S)}}}}\text{+ 4N}{{\text{O}}_{{\text{2(g)}}}}\text{+ }{{\text{O}}_{{\text{2(g)}}}}[/latex]
.
Lead nitrate is a colourless solid, and when it is heated, brown fumes of nitrogen dioxide will be produced, and lead oxide which is a yellow solid will be left behind. - Hydroxides: Metal hydroxides decompose on heating to yield metal oxides and water. Sodium hydroxide decomposes to produce sodium oxide and water:
[latex]\scriptsize \displaystyle \text{2NaO}{{\text{H}}_{{\left( \text{s} \right)}}}\to \text{N}{{\text{a}}_{\text{2}}}{{\text{O}}_{{\left( \text{s} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}[/latex]
.
Some unstable acids decompose to produce nonmetal oxides and water. Carbonic acid decomposes easily at room temperature into carbon dioxide and water:
[latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{{\text{3}\left( {\text{aq}} \right)}}}\to \text{C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}[/latex]
Rules for thermal decomposition:
- Carbonates decompose into carbon dioxide and an oxide.
- Chlorates decompose into oxygen gas and a chloride.
- Hydroxides decompose into water and an oxide.
- Acids containing oxygen decompose into water and a molecular oxide.
- Oxides decompose into oxygen and another element.
| Metal | Chlorate ([latex]\scriptsize \text{Cl}{{\text{O}}_{\text{3}}}[/latex]) | Nitrate ([latex]\scriptsize \text{N}{{\text{O}}_{3}}[/latex]) | Carbonate ([latex]\scriptsize \text{C}{{\text{O}}_{\text{3}}}[/latex]) | Hydroxide ([latex]\scriptsize \text{OH}[/latex]) |
| Potassium Sodium |
metal chlorate→ metal chloride + oxygen | metal nitrate → metal oxide + nitrogen dioxide + oxygen | no decomposition | no decomposition |
| Calcium Magnesium Aluminium Zinc Iron Lead Copper |
metal chlorate→ metal chloride + oxygen | metal nitrate → metal oxide + nitrogen dioxide + oxygen | metal carbonate → metal oxide + carbon dioxide | metal hydroxide → metal oxide + water |
| Silver Gold |
metal chlorate→ metal chloride + oxygen | metal nitrate → metal oxide + nitrogen dioxide + oxygen | meta carbonate → metal oxide + carbon dioxide | does not exist |
Note
To find out more about this, you can watch a video about the decomposition of carbonates, called Heating carbonates C0050, by Nigel Baldwin.
Electrolytic decomposition
In electrolytic decomposition, a substance will decompose when an electric current is passed through an aqueous solution of a substance.
Passing an electric current through water decomposes it to form hydrogen and oxygen:
[latex]\scriptsize \displaystyle \text{2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{2}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
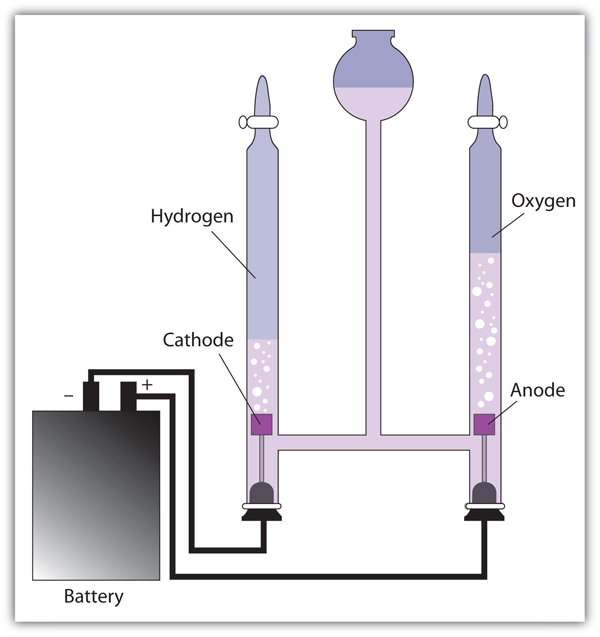
Sodium chloride can be decomposed as molten sodium chloride to give off sodium and chlorine, but it is easier to decompose when it is in an aqueous solution to form sodium hydroxide, chlorine and hydrogen gases:
[latex]\scriptsize \displaystyle \text{2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\text{ + 2NaCl }\to \text{ }{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{ + C}{{\text{l}}_{\text{2}}}_{{\text{(g) }}}\text{+ 2NaO}{{\text{H}}_{{\text{(s)}}}}[/latex]
Photo decomposition
Photo decomposition is the decomposition of substances in light. As mentioned earlier hydrogen peroxide decomposes in the presence of light, which is why it is usually stored in a brown bottle.
Silver chloride decomposes in light to form silver and releases chlorine gas:
[latex]\scriptsize \text{2AgC}{{\text{l}}_{{\text{(s)}}}}\to \text{2A}{{\text{g}}_{{\text{(s)}}}}\text{+C}{{\text{l}}_{{\text{2(g)}}}}[/latex]
Application of decomposition reactions
One major application of decomposition reactions is in the extraction of metals from their ores. For example, zinc can be obtained from calamine in a similar manner to how sodium can be obtained from sodium chloride. Other industrial applications include:
- the production of calcium oxide or quicklime
- the production of lithium oxide
- the preparation of oxygen and carbon dioxide.
| Combination reactions | Decomposition reactions | |
| Two or more reactant compounds are involved in combination reactions. | A single compound is involved in a decomposition reaction. | |
| Products | result in a single product | result in several products |
| Chemical bonds | result in bonding of atoms to produce the single end product | chemical bonds are broken down to form two or more end products |
| Molecules | cause simple molecules to react and produce complex molecules | cause complex molecules to break down into simple molecules |
Exercise 1.4
- Why are decomposition reactions mostly endothermic in nature?
- State which of the following are examples of decomposition reactions:
- Calcium carbonate is heated. Calcium oxide is formed, and carbon dioxide is given off.
- Magnesium oxide is heated to give magnesium and oxygen.
- You burn a piece of toast. It turns black.
The full solutions can be found at the end of the unit.
Summary
In this unit you have learnt the following:
- A composition reaction produces a single substance from multiple reactants.
- A decomposition reaction produces multiple products from a single reactant.
- A displacement reaction happens when a more reactive element takes the place of a less reactive element in a chemical compound.
- The reactivity series is a list of metals according to their ability to react with oxygen, water, and acids.
- Combination reactions and decomposition reactions are simple reactions which are the opposite of each other.
- The main difference between combination and decomposition reactions is that a combination reaction involves the combination of two or more reactant molecules to result in a single end-product whereas decomposition reaction involves the breakdown of a single compound into two or more products.
Unit 1: Assessment
Suggested time to complete: 20 minutes
- How does a combination reaction differ from a decomposition reaction?
- Write and name the general equation of each type of chemical reaction.
- For the following reactions, state which are examples of combination reactions:
- [latex]\scriptsize \displaystyle \text{NaCl + AgN}{{\text{O}}_{\text{3}}}\text{ }\to \text{ AgCl + NaN}{{\text{O}}_{\text{3}}}[/latex]
- [latex]\scriptsize \displaystyle \text{CaO + C}{{\text{O}}_{\text{2}}}\text{ }\to \text{ CaC}{{\text{O}}_{\text{3}}}[/latex]
- [latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}\text{ + C}{{\text{l}}_{\text{2}}}\text{ }\to \text{2HCl}[/latex]
- [latex]\scriptsize \displaystyle \text{2HBr + C}{{\text{l}}_{\text{2}}}\text{ }\to \text{2HCl + B}{{\text{r}}_{\text{2}}}[/latex]
- For the following reactions, state which are examples of decomposition reactions:
- [latex]\scriptsize \displaystyle \text{HCl + NaOH}\to \text{NaCl + }{{\text{H}}_{\text{2}}}\text{O}[/latex]
- [latex]\scriptsize \displaystyle \text{CaC}{{\text{O}}_{\text{3}}}\to \text{CaO + C}{{\text{O}}_{\text{2}}}[/latex]
- [latex]\scriptsize \displaystyle \text{3}{{\text{O}}_{\text{2}}}\to \text{ 2}{{\text{O}}_{\text{3}}}[/latex]
- [latex]\scriptsize \displaystyle \text{2KCl}{{\text{O}}_{\text{3}}}\to \text{2KCl + 3}{{\text{O}}_{\text{2}}}[/latex]
- Predict the products that will result when these reactants combine and write a balanced chemical equation for each:
- [latex]\scriptsize \displaystyle \text{Fe + CuS}{{\text{O}}_{\text{4}}}\to[/latex]
- [latex]\scriptsize \displaystyle \text{B}{{\text{r}}_{\text{2}}}\text{ + MgC}{{\text{l}}_{\text{2}}}\to[/latex]
- [latex]\scriptsize \displaystyle \text{Mg + AlC}{{\text{l}}_{\text{3}}}\text{ }\to[/latex]
- aluminium bromide ([latex]\scriptsize \text{AlB}{{\text{r}}_{\text{3}}}[/latex]) + chlorine
- sodium iodide ([latex]\scriptsize \text{NaI}[/latex]) + bromine
- calcium + hydrochloric acid
- magnesium + nitric acid ([latex]\scriptsize \text{HN}{{\text{O}}_{\text{3}}}[/latex])
- potassium + water
- What is the reactivity series and how does it relate to chemical reactions?
The full solutions can be found at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- [latex]\scriptsize \displaystyle \text{0}\text{.00 g }{{\text{K}}_{\text{2}}}\text{O}[/latex]. Potassium reacts with oxygen to form [latex]\scriptsize \displaystyle {{\text{K}}_{\text{2}}}{{\text{O}}_{\text{2}}}[/latex] and [latex]\scriptsize \displaystyle \text{K}{{\text{O}}_{\text{2}}}[/latex] only.
- Xenon
- [latex]\scriptsize \displaystyle \text{4A}{{\text{l}}_{{\left( \text{s} \right)\text{ }}}}\text{+ 3}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{ 2A}{{\text{l}}_{\text{2}}}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}[/latex]
- High temperatures – It takes energy to cause those reactions.
- Li generally forms [latex]\scriptsize \displaystyle \text{L}{{\text{i}}_{\text{2}}}\text{O}\text{.}[/latex] Na usually forms [latex]\scriptsize \displaystyle \text{N}{{\text{a}}_{\text{2}}}{{\text{O}}_{\text{2}}}[/latex]. The rest of group 1 elements tend to form compounds in the form of [latex]\scriptsize \displaystyle {{\text{M}}_{\text{2}}}\text{O}[/latex] where M represents the letter for the group 1 element.
Exercise 1.2
- a) Lithium is found in group 1 of the periodic table and is therefore a very reactive metal.
- c) Magnesium is more reactive than copper, so it will displace it from a solution of copper sulfate.
- a) Iron oxide had its oxygen removed in this reaction, so it was reduced.
- a) The lead oxide was the source of the oxygen in this reaction, so it was the oxidising agent.
Exercise 1.3
- .
- No reaction, beryllium does not react with water.
- No reaction, noble gases do not react with water.
- [latex]\scriptsize \displaystyle \text{L}{{\text{i}}_{\text{2}}}{{\text{O}}_{{\left( \text{s} \right)}}}\text{+2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\to \text{2LiO}{{\text{H}}_{{\left( {\text{aq}} \right)}}}[/latex]
- .
- True
- False
- No reaction occurs because there is a protective layer of aluminium oxide which prevents any reaction.
Exercise 1.4
- Most decomposition reactions require energy either in the form of heat, light, or electricity. Absorption of energy causes the breaking of the bonds present in the reacting substance which decomposes to give the product.
- b and c
Unit 1: Assessment
- In a combination reaction, two or more substances combine to form a single product. However, in a decomposition reaction, a single substance breaks down to give two or more substances. Therefore, the two are opposite to each other. Generally, combination reactions are mostly exothermic, while decomposition reactions are endothermic.
- Combination: [latex]\scriptsize \displaystyle \text{A + B }\to \text{AB}[/latex]
Decomposition: [latex]\scriptsize \displaystyle \text{AB }\to \text{A + B}[/latex]
Single displacement: [latex]\scriptsize \displaystyle \text{AB + C }\to \text{ AC + B}[/latex] - .
- yes
- yes
- yes
- no
- .
- no
- yes
- no
- yes
- .
- [latex]\scriptsize \displaystyle \text{Fe + CuS}{{\text{O}}_{\text{4}}}\to \text{FeS}{{\text{O}}_{\text{4}}}\text{+Cu}[/latex]
- [latex]\scriptsize \displaystyle \text{B}{{\text{r}}_{\text{2}}}\text{ + MgC}{{\text{l}}_{\text{2}}}\to[/latex] No reaction will occur because bromine is less reactive than chlorine.
- [latex]\scriptsize \displaystyle \text{3 Mg + 2AlC}{{\text{l}}_{\text{3}}}\text{ }\to 2\text{Al + 3MgC}{{\text{l}}_{\text{2}}}[/latex]
- [latex]\scriptsize \displaystyle \text{2AlB}{{\text{r}}_{\text{3}}}\text{ + 3C}{{\text{l}}_{\text{2}}}\text{ }\to \text{2AlC}{{\text{l}}_{\text{3}}}\text{ + 3B}{{\text{r}}_{\text{2}}}[/latex]
- [latex]\scriptsize \displaystyle \text{2NaI + B}{{\text{r}}_{\text{2}}}\text{ }\to \text{ 2NaBr + }{{\text{I}}_{\text{2}}}[/latex]
- [latex]\scriptsize \displaystyle \text{Ca + 2HCl }\to \text{ CaC}{{\text{l}}_{\text{2}}}\text{ + }{{\text{H}}_{\text{2}}}\text{ }[/latex]
- [latex]\scriptsize \displaystyle \text{Mg + 2HN}{{\text{O}}_{\text{3}}}\text{ }\to \text{ Mg}{{\left( {\text{N}{{\text{O}}_{\text{3}}}} \right)}_{\text{2}}}\text{ + }{{\text{H}}_{\text{2}}}[/latex]
- [latex]\scriptsize \displaystyle \text{2K + 2}{{\text{H}}_{\text{2}}}\text{O }\to \text{ 2KOH + }{{\text{H}}_{2}}[/latex]
- The reactivity series is a list of metals (or halogens) in order of decreasing reactivity. It is used to predict which reaction will occur. For example, a metal with a higher activity will replace a metal with a lower activity in a single displacement reaction. The same is true for halogens.
Media Attributions
- Fig 1 © DHET is licensed under a CC BY (Attribution) license
- Fig 2 © Rice University is licensed under a CC BY (Attribution) license
- Fig 3 © Capt. John Yossarian is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 4 © DHET is licensed under a CC BY (Attribution) license
- Fig 5 © DHET is licensed under a CC BY (Attribution) license
- Fig 6 © DHET is licensed under a CC BY (Attribution) license
- Fig 7 © Chemlegen is licensed under a CC0 (Creative Commons Zero) license
- Fig 8 © Chemlegen is licensed under a CC0 (Creative Commons Zero) license
- Fig 9 © Chemlegen is licensed under a CC0 (Creative Commons Zero) license
- Fig 10 © DHET is licensed under a CC BY (Attribution) license
- Fig 11 © DHET is licensed under a CC BY (Attribution) license
- Fig 12 © LibreTextx is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 13 © Lumen Learning is licensed under a CC BY (Attribution) license
a chemical reaction in which more than one reactant gives only one product
any aqueous solution which has a pH > 7.0
any aqueous solution which has a pH < 7.0
chemical compounds containing a metal from group 1 and one or more oxygen atoms
any of two or more physical forms in which an element can exist
the loss of electrons
the gain of electrons
a type of chemical reaction where one species is reduced and the other is oxidised. Reduction is the gaining of electrons and oxidation is the loss of electrons.
a chemical reaction where a more reactive element takes the place of a less reactive element in a chemical compound
an exothermic reaction between the metal and metal oxide
When a species is oxidised it loses electrons.
When a species is reduced it gains electrons
any compound of hydrogen with another element
chemical compounds consisting of a halogen and another element, especially a strongly electropositive metal such as sodium or potassium
chemical reactions where one single compound splits into multiple simpler substances
