Chemical Change: Identify, analyse and apply energy changes
Unit 1: Energy changes
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Describe and identify endothermic reactions.
- Describe and identify exothermic reactions.
- Describe and identify enthalpy.
- Describe and identify activation energy.
What you should know
Before you start this unit, make sure you can:
- Understand bonding. Refer to level 2 subject outcome 5.4 unit 3 to revise this.
Introduction
Parts of the text in this unit were sourced from Siyavula Physical Science Gr 11 Learner’s Book, Chapter 12, released under a CC-BY licence.
Enthalpy is a measure of the total amount of thermal energy in a system which is useful to know as it can be used to do work. Most chemical reactions involve enthalpy changes. Energy is absorbed to break bonds in the reactants and released when new bonds are formed to make the products. The comparison of the input and output energies results in either an overall release of thermal energy – exothermic reactions – or an overall absorption in thermal energy – endothermic reactions.
Energy changes
When a chemical reaction occurs, bonds in the reactants break, while new bonds form in the formation of the product. For example, when hydrogen reacts with oxygen to form water. The equation for this reaction is: [latex]\scriptsize \displaystyle \text{2}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}[/latex].
The bond between the two hydrogen atoms in the H2 molecule will break, so will the bond between the oxygen atoms in the O2 molecule. New bonds will form between the two hydrogen atoms and the single oxygen atom in the water molecule that is formed as the product.
For bonds to break, energy must be absorbed. When new bonds form, energy is released.
The energy that is required to break a bond or that is released when a bond is formed is called the or bond dissociation energy. Bond energies are measured in units [latex]\scriptsize \displaystyle \text{kJ}\text{.mo}{{\text{l}}^{{\text{-1}}}}[/latex].
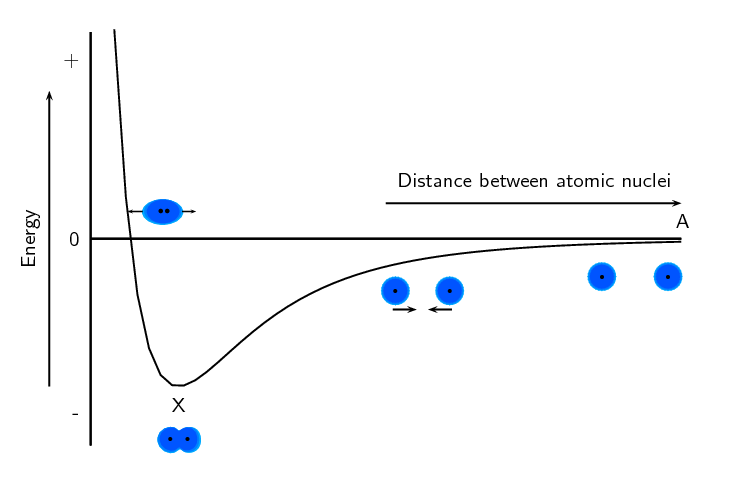
We can use the diagram in figure 1 to understand why bond breaking requires energy and bond making releases energy. Point X on the diagram is at the lowest energy. When a bond breaks, the atoms move apart and the distance between them increases (i.e. the atom moves to the right on the x-axis or from point X to point A). Looking at the diagram we see that when this happens, the energy increases (i.e. the energy at point A is greater than the energy at point X). As a result, when a bond breaks energy is needed.
When a bond forms the atoms move closer together and the distance between them decreases (i.e. the atom moves to the left on the x-axis or from point A to point X). Looking at the diagram we see that when this happens, the energy decreases (i.e. the energy at point X is less than the energy at point A). As a result, when a bond forms energy is released.
Let’s look at the example of hydrogen reacting with oxygen to form water:
[latex]\scriptsize \displaystyle \text{2}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{+}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}[/latex]
Energy is needed to break the bonds in the hydrogen molecule and to break the bonds in the oxygen molecule. Also, energy is released when hydrogen and oxygen bond to form water. When we look at the entire reaction and consider both bond breaking and bond forming, we need to look at the change of the system.
Endothermic and exothermic reactions
Let’s look at an activity to explore endothermic and exothermic reactions.
Activity 1.1: Explore endothermic and exothermic reactions
Time required: 30 minutes
What you need:
- juice from a lemon
- sodium bicarbonate
- a polystyrene cup
- a lid for the cup
- a thermometer
- a glass stirring rod
- scissors
You can get polystyrene cups with lids from coffee shops or fast food stores. Cardboard cups will also work fine. Some of the lids will have a hole for a straw, which is useful for this experiment, and you can find sachets of bicarbonate of soda and citric acid at a supermarket.
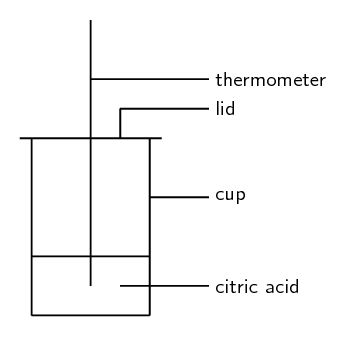
What to do:
- If your lid does not have a hole for a straw, then cut a small hole into the lid. This is for the thermometer.
- Mix [latex]\scriptsize \displaystyle 2[/latex] teaspoons of citric acid with [latex]\scriptsize \displaystyle 5\text{ mls}[/latex] of water to form a paste, replace the lid and measure and record the temperature using the thermometer.
- Stir in a teaspoon of sodium bicarbonate (NaHCO3), then cover the cup again. Immediately record the temperature.
- Take a temperature reading every two minutes after that. Record your results in a table in your notebook.
.
ResultsTime (mins) Temperature (oC) [latex]\scriptsize 0[/latex] [latex]\scriptsize 2[/latex] [latex]\scriptsize 4[/latex] [latex]\scriptsize 6[/latex] - Plot your temperature results on a graph of time (x-axis) against temperature (y-axis).
- What happens to the temperature during this reaction?
- Did the temperature increase or decrease?
What did you find?
The lemon juice contains citric acid ([latex]\scriptsize {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{7}}}[/latex]) which reacts with the sodium bicarbonate ([latex]\scriptsize \text{NaHC}{{\text{O}}_{\text{3}}}[/latex]) to produce sodium citrate, water, and carbon dioxide. This reaction gives out heat so as the reaction occurs you should have noticed that the temperature increased.
[latex]\scriptsize \displaystyle {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{{\text{7}\left( {\text{aq}} \right)}}}\text{+ 3NaHC}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}\to \text{3C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{+ 3}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\text{(l)}}}}\text{+ N}{{\text{a}}_{\text{3}}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{{\text{O}}_{{\text{7}\left( {\text{aq}} \right)}}}[/latex]
In some reactions, the energy that must be absorbed to break the bonds in the reactants, is less than the energy that is released when the new bonds of the products are formed. This means that in the overall reaction, energy is released as either heat or light. This type of reaction is called an .
Another way of describing an exothermic reaction is that it is one in which the energy of the products is less than the energy of the reactants, because energy has been released during the reaction. We can represent this using the following general formula:
reactants → products + energy
As in the experiment, the temperature of the surroundings will increase because of the overall release of thermal energy.
In other reactions, the energy that must be absorbed to break the bonds in the reactants, is more than the energy that is released when the new bonds in the products are formed. This means that in the overall reaction, energy must be absorbed from the surroundings. This type of reaction is known as an .
Another way of describing an endothermic reaction is that it is one in which the energy of the products is greater than the energy of the reactants, because energy has been absorbed during the reaction. This can be represented by the following general formula:
reactants + energy → products
In an endothermic reaction, there will be a decrease in the temperature of the surroundings because of the overall absorption of thermal energy from the surroundings which is now in the form of chemical energy in the bonds of the products.
The difference in energy between the reactants and the products is known as the heat of the reaction. It is also sometimes referred to as the enthalpy change of the system. This is represented using [latex]\scriptsize \displaystyle \Delta H[/latex].
[latex]\scriptsize \displaystyle \Delta[/latex] is read as delta and means a change in.
Examples of exothermic and endothermic reactions
The burning of fuel is an example of a combustion reaction, and we as humans rely heavily on this process for our energy requirements. The following equations describe the combustion of a hydrocarbon such as petrol ([latex]\scriptsize \displaystyle {{\text{C}}_{8}}{{\text{H}}_{{18}}}[/latex]):
fuel + oxygen → heat + water + carbon dioxide
[latex]\scriptsize \displaystyle \text{2}{{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{18}\left( \text{l} \right)}}}\text{+ 25}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{16C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{+ 18}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}\text{+ heat}[/latex]
We burn fuels (such as paraffin, coal, propane, and butane) for energy, and because the chemical changes that take place during the reaction release huge amounts of energy, we can use this released energy for things like power and electricity.
Respiration is the chemical reaction that happens in our bodies to produce energy for our cells. The equation below describes what happens during this reaction:
[latex]\scriptsize \displaystyle {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{{\text{6}\left( \text{s} \right)}}}\text{+ 6}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{6C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{+ 6}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\text{+ energy}[/latex]
In the reaction, glucose reacts with oxygen from the air that we breathe in, to form carbon dioxide water and energy. The energy that is produced allows the cell to carry out its functions efficiently.
It is not the food itself that provides you with energy, but the exothermic reaction that takes place when compounds within the food react with the oxygen you have inhaled.
Photosynthesis is the chemical reaction that takes place in green plants, which uses energy from the sun to change carbon dioxide and water into food that the plant needs to survive, and which other organisms can eat so that they too can survive. The equation for this reaction is:
[latex]\scriptsize \displaystyle \text{6C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{+ 6}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\text{+ energy}\to {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}_{{\left( \text{s} \right)}}\text{+ 6}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Photosynthesis is an endothermic reaction. Energy in the form of sunlight is absorbed during the reaction.
In industry, the breakdown of limestone into quicklime and carbon dioxide is important.
[latex]\scriptsize \displaystyle \text{CaC}{{\text{O}}_{{\text{3}\left( \text{s} \right)}}}\text{ + heat }\to \text{Ca}{{\text{O}}_{{\left( \text{s} \right)}}}\text{+ C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}[/latex]
Quicklime ([latex]\scriptsize \displaystyle \text{CaO}[/latex]) can be used to make steel from iron and to neutralise soils that are too acid. However, the limestone must be heated in a kiln at a temperature of over before the decomposition reaction will take place.
Exercise 1.1
- State whether energy is taken in or released in each of the following situations:
- The bond between hydrogen and chlorine in a molecule of hydrogen chloride breaks.
- A bond is formed between hydrogen and fluorine to form a molecule of hydrogen fluoride.
- A molecule of nitrogen ([latex]\scriptsize \displaystyle {{\text{N}}_{\text{2}}}[/latex]) is formed.
- A molecule of carbon dioxide breaks apart.
- State whether the following descriptions are used to describe an endothermic or an exothermic reaction:
- Reactants react to give products and energy.
- The energy that must be absorbed to break the bonds in the reactants is greater than the energy that is released when the products form.
- The energy of the products is found to be greater than the energy of the reactants for this type of reaction.
- Heat or light must be absorbed from the surroundings before this type of reaction takes place.
The full solutions can be found at the end of the unit.
The heat of reaction
The heat of the reaction is represented by the symbol [latex]\scriptsize \displaystyle \Delta H[/latex], where:
[latex]\scriptsize \displaystyle \Delta H={{E}_{{prod}}}-{{E}_{{react}}}[/latex]
In an exothermic reaction, [latex]\scriptsize \displaystyle \Delta H[/latex] is less than zero (or negative) because the energy of the reactants is greater than the energy of the products. Overall, energy is released in the reaction. For example when hydrogen and chlorine react to form hydrogen chloride:
[latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}_{{\left( \text{g} \right)}}\text{+ C}{{\text{l}}_{{\text{2}\left( \text{g} \right)}}}\text{ }\to \text{2HC}{{\text{l}}_{{\left( \text{g} \right)}}}\text{ }\Delta H \lt 0[/latex]
In an endothermic reaction, [latex]\scriptsize \displaystyle \Delta H[/latex] is greater than zero (or positive) because the energy of the reactants is less than the energy of the products. Energy is absorbed in the reaction. For example when carbon and water react to form carbon monoxide and hydrogen:
[latex]\scriptsize \displaystyle {{\text{C}}_{{\left( \text{s} \right)}}}\text{+ }{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{l} \right)}}}\text{ }\to \text{C}{{\text{O}}_{{\left( \text{g} \right)}}}\text{+ }{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{ }\Delta H \gt 0[/latex]
Writing equations
The units for [latex]\scriptsize \displaystyle \Delta H[/latex] are [latex]\scriptsize \displaystyle \text{kJ}\text{.mo}{{\text{l}}^{{\text{-1}}}}[/latex]. In other words, the [latex]\scriptsize \displaystyle \Delta H[/latex] value gives the amount of energy that is absorbed or released per mole of product that is formed. Units can also be written as [latex]\scriptsize \displaystyle \text{kJ}[/latex], which then gives the total amount of energy that is released or absorbed when the product forms.
For the exothermic reaction between carbon and oxygen, we can write:
[latex]\scriptsize \displaystyle \begin{align*}{{\text{C}}_{{\left( \text{s} \right)}}}\text{+ }{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{ }\to \text{C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{ }&\Delta H=-393\text{ kJ}\text{.mo}{{\text{l}}^{{\text{-1}}}}\\& or\\{{\text{C}}_{{\left( \text{s} \right)}}}\text{+ }{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to \text{C}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{ }&+393\text{ kJ}\text{.mo}{{\text{l}}^{{\text{-1}}}}\end{align*}[/latex]
For the endothermic reaction between carbon and hydrogen we can write:
[latex]\scriptsize \displaystyle \begin{align*}{{\text{C}}_{{\left( \text{s} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}\to {{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{+C}{{\text{O}}_{{\left( \text{g} \right)}}}&\text{ }\!\!\Delta\!\!\text{ H}=+131\text{ kJ}\text{.mo}{{\text{l}}^{{\text{-1}}}}\\& or\\{{\text{C}}_{{\left( \text{s} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}\text{+} 131\text{ kJ}\text{.mo}{{\text{l}}^{{\text{-1}}}}& \to {{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{+ C}{{\text{O}}_{{\left( \text{g} \right)}}}\end{align*}[/latex]
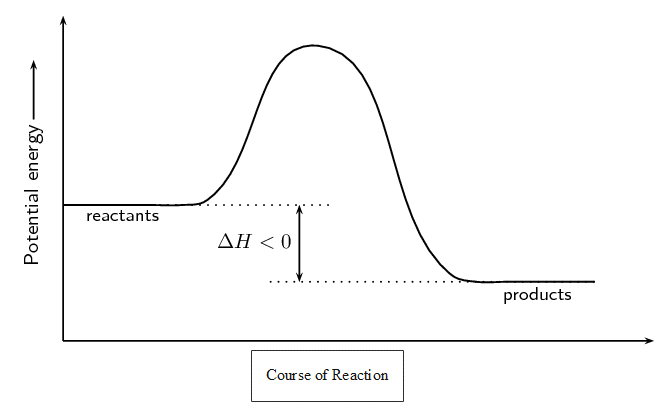
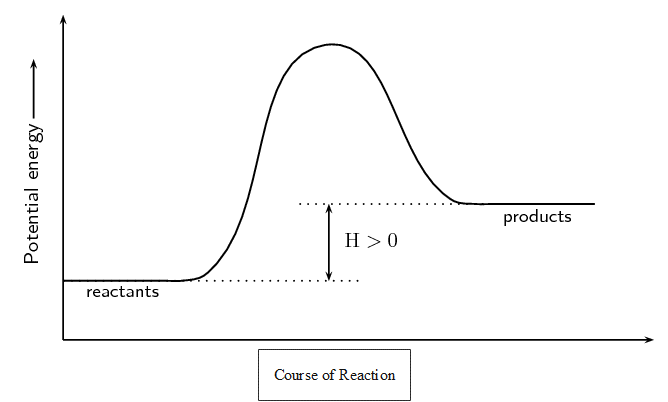
The energy changes during exothermic and endothermic reactions can be plotted on a graph:
Activation energy
Activation energy is the minimum amount of energy needed to start a chemical reaction. The reaction will not take place until the system has the minimum amount of energy added to it. The activation energy is the difference between the energy of the reactants and the energy required to break bonds in the reactants.
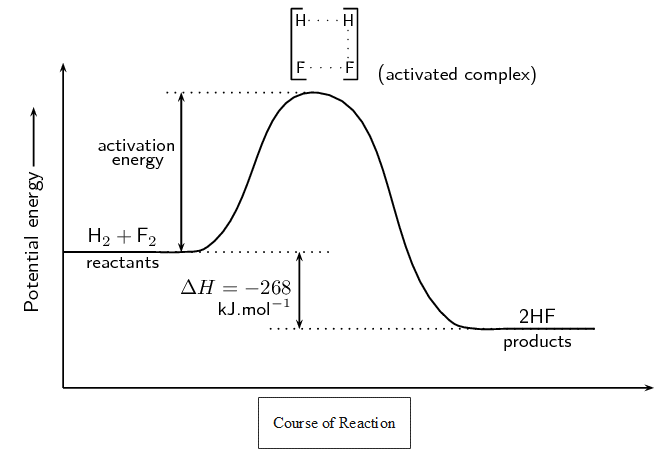
NASA uses the reaction between hydrogen and fluorine as rocket fuel because of the amount of energy released. It is an exothermic reaction.
[latex]\scriptsize \displaystyle {{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\text{+}{{\text{F}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2H}{{\text{F}}_{{\left( \text{g} \right)}}}[/latex]
The reaction between hydrogen and fluorine needs energy to proceed, and this is the activation energy. To form the product the bond between [latex]\scriptsize \displaystyle \text{H}[/latex] and [latex]\scriptsize \displaystyle \text{H}[/latex] in [latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}[/latex] must break. The bond between [latex]\scriptsize \displaystyle \text{F}[/latex] and [latex]\scriptsize \displaystyle \text{F}[/latex] in [latex]\scriptsize \displaystyle {{\text{F}}_{\text{2}}}[/latex] must also break. This unstable, intermediate state when all the atoms are separates is called the activated complex or transition state. The activated complex lasts for only a short time. After this short time one of two things will happen: the original bonds will reform, or different bonds will form and a new product is made.
In this example, the final product is [latex]\scriptsize \displaystyle \text{HF}[/latex] and it has a lower energy than the reactants. The reaction is exothermic and [latex]\scriptsize \displaystyle \Delta H[/latex] is negative.
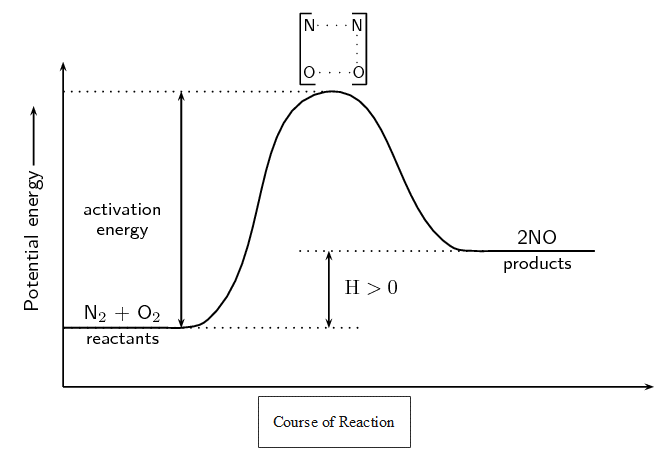
In endothermic reactions, the final products have a higher energy than the reactants. An energy diagram is shown in figure 5 for the endothermic reaction:
[latex]\scriptsize \displaystyle {{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\text{+}{{\text{N}}_{{\text{2}\left( \text{g} \right)}}}\to \text{2N}{{\text{O}}_{{\left( \text{g} \right)}}}[/latex]
Example 1.1
Using the graph below, answer the following questions:
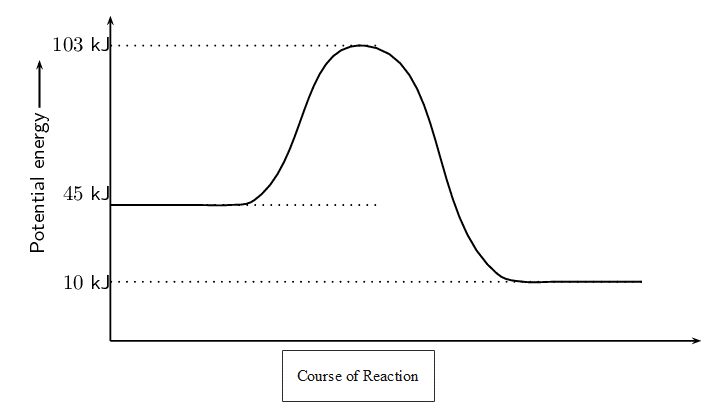
- Calculate [latex]\scriptsize \displaystyle \Delta H[/latex].
- Determine if this reaction is exothermic or endothermic.
- Calculate the activation energy.
Solutions
- [latex]\scriptsize \displaystyle \Delta H[/latex] is found by subtracting the energy of the reactants from the energy of the products. We find the energy of the reactants and the products from the graph.
.
[latex]\scriptsize \displaystyle \Delta H[/latex] = energy of products − energy of reactants = [latex]\scriptsize \displaystyle 10\text{ kJ}-45\text{ kJ}=-35\text{ kJ}[/latex] - The reaction is exothermic since [latex]\scriptsize \displaystyle \Delta H \lt 0[/latex].
.
We also note that the energy of the reactants is greater than the energy of the products. - The activation energy is found by subtracting the energy of the reactants from the energy of the activated complex. Again we can read the energy of the reactants and activated complex off the graph.
.
activation energy = energy of activated complex − energy of reactants = [latex]\scriptsize \displaystyle 103\text{ kJ}-45\text{ kJ}=58\text{ kJ}[/latex]
Summary
In this unit you have learnt the following:
- When a reaction occurs, bonds in the reactants break and new bonds form to make the products. These changes involve energy.
- When bonds break, energy is absorbed and when new bonds form, energy is released.
- The bond energy is a measure of bond strength in a chemical bond. It is the amount of energy that is needed to break the chemical bond between two atoms, or the amount of energy released when that bond is formed.
- Enthalpy is a measure of the total energy of a chemical system for a given pressure and is given the symbol [latex]\scriptsize \displaystyle \text{H}[/latex].
- If the energy that is needed to break the bonds is less than the energy that is released when new bonds form, then the reaction is exothermic. The energy of the products is less than the energy of the reactants.
- An exothermic reaction is one that releases energy in the form of heat or light. The temperature of the surroundings will increase.
- If the energy that is needed to break the bonds is more than the energy that is released when new bonds form, then the reaction is endothermic. The energy of the products is greater than the energy of the reactants.
- An endothermic reaction is one that absorbs energy in the form of heat or light. The temperature of the surroundings will decrease.
- Photosynthesis and the thermal decomposition of limestone are both examples of endothermic reactions.
- Combustion reactions and respiration are both examples of exothermic reactions.
- The difference in energy between the reactants and the product is called the heat of reaction and has the symbol [latex]\scriptsize \displaystyle \Delta H[/latex].
- [latex]\scriptsize \displaystyle \Delta H[/latex] is calculated using: [latex]\scriptsize \displaystyle \Delta H={{E}_{{prod}}}-{{E}_{{react}}}[/latex]
- In an endothermic reaction, [latex]\scriptsize \displaystyle \Delta H[/latex] is a positive number (greater than 0). In an exothermic reaction, [latex]\scriptsize \displaystyle \Delta H[/latex] will be negative (less than 0).
- Chemical reactions will not take place until the system has some minimum amount of energy added to it.
- The activation energy is the minimum amount of energy that is needed to start a chemical reaction.
Unit 1: Assessment
Suggested time to complete: 20 minutes
- For each of the following, give one word or term for the description.
- The minimum amount of energy that is needed for a reaction to proceed.
- A measure of the bond strength in a chemical bond.
- A type of reaction where [latex]\scriptsize \displaystyle \Delta H[/latex] is less than zero.
- A type of reaction that requires heat or light to proceed.
- Carbon reacts with water according to the following equation:
[latex]\scriptsize \displaystyle {{\text{C}}_{{\left( \text{s} \right)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{{\left( \text{g} \right)}}}\to \text{C}{{\text{O}}_{{\left( \text{g} \right)}}}\text{+}{{\text{H}}_{{\text{2}\left( \text{g} \right)}}}\Delta H \gt 0[/latex]
Is this reaction endothermic or exothermic? Give a reason for your answer. - Refer to the graph below and then answer the questions that follow:
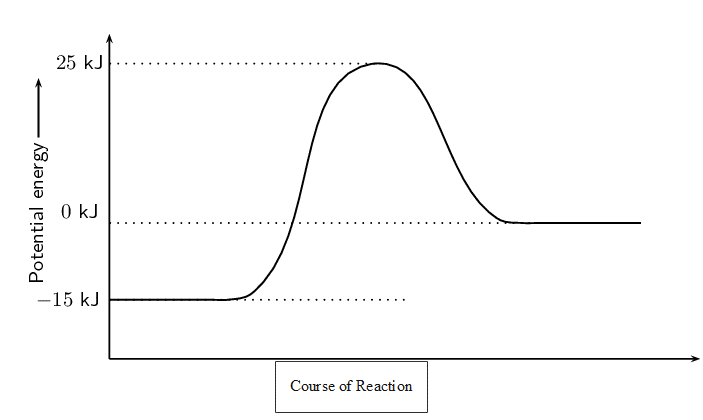
- What is the energy of the reactants?
- What is the energy of the products?
- Calculate [latex]\scriptsize \displaystyle \Delta H[/latex].
- What is the activation energy for this reaction?
- Consider the following chemical reaction:
[latex]\scriptsize \displaystyle \text{2N}{{\text{O}}_{{\text{2}\left( \text{g} \right)}}}\to {{\text{N}}_{\text{2}}}{{\text{O}}_{{\text{4}\left( \text{g} \right)}}}\text{ }\Delta H \lt 0[/latex]
Which one of the following graphs best represents the changes in potential energy that take place during the production of [latex]\scriptsize \displaystyle {{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}[/latex]?
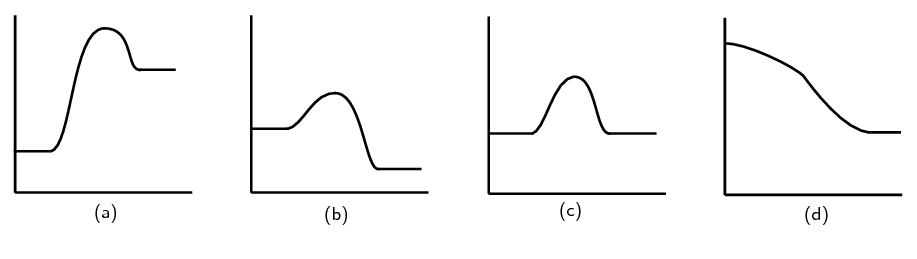
The full solutions can be found at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- .
- This is bond breaking and so energy is taken in.
- This is bond forming and so energy is released.
- A bond is formed and so energy is released.
- A bond is broken and so energy is taken in.
- .
- Exothermic
- Endothermic
- Endothermic
- Endothermic
Unit 1: Assessment
- .
- activation energy
- bond energy
- an exothermic reaction
- an endothermic reaction
- Endothermic, as [latex]\scriptsize \displaystyle \Delta H \gt 0[/latex]
- .
- [latex]\scriptsize -15\text{ KJ}[/latex]
- [latex]\scriptsize 0\text{ KJ}[/latex]
- [latex]\scriptsize \displaystyle \Delta H[/latex] = energy of products − energy of reactants = [latex]\scriptsize \displaystyle \text{0 kJ-}\left( {\text{-15 kJ}} \right)\text{=15 kJ}[/latex]
- activation energy = energy of activated complex − energy of reactants = [latex]\scriptsize \displaystyle \text{25 kJ-}\left( {\text{-15 kJ}} \right)\text{=40 kJ}[/latex]
- b) is correct. The reaction is exothermic ([latex]\scriptsize \displaystyle \Delta H \gt 0[/latex]), so the energy of the reactants must be greater than the energy of the products.
Media Attributions
- Fig 1 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 2 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 3 © DHET is licensed under a CC BY (Attribution) license
- Fig 4 © DHET is licensed under a CC BY (Attribution) license
- Fig 5 © DHET is licensed under a CC BY (Attribution) license
- Fig 6 © DHET is licensed under a CC BY (Attribution) license
- Fig 7 © DHET is licensed under a CC BY (Attribution) license
- Fig 8 © DHET is licensed under a CC BY (Attribution) license
- Fig 9 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
a measure of bond strength in a chemical bond; it is the amount of energy that is needed to break the chemical bond between two atoms, or the amount of energy released when that bond is formed
a measure of the total energy of a chemical system for a given pressure, and is given the symbol H
a reaction in which there is an overall release of energy in the form of heat or light
a reaction in which there is an overall absorption of energy in the form of heat or light
