Mechanical Properties of matter: State, analyse and apply principles of atomic combinations: molecular structure
Unit 2: Electronegativity and polarity
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Define electronegativity of atoms and apply it to explain the polarity of chemical bonds.
What you should know
Before you start this unit, make sure you can:
- Understand molecules and molecular structure. Refer to level 3 subject outcome 5.2 unit 1 to revise this.
Introduction
Parts of the text in this unit were sourced from Siyavula Physical Science Gr 11 Learner’s Book, Chapter 3, released under a CC-BY licence.
In this unit you will learn about the electronegativity of atoms and apply this to explain the polarity of chemical bonds. Electronegativity is the tendency of an atom to attract electrons to itself.
Electronegativity
can be defined as a measure of the attraction of a bonded atom for pairs of electrons and is measured on the Pauling scale. Electronegativity increases up a group and across a period in the periodic table, with fluorine being the most electronegative element. Non-metals have a higher electronegativity than metals. This means that metals are less likely to hold onto electrons.
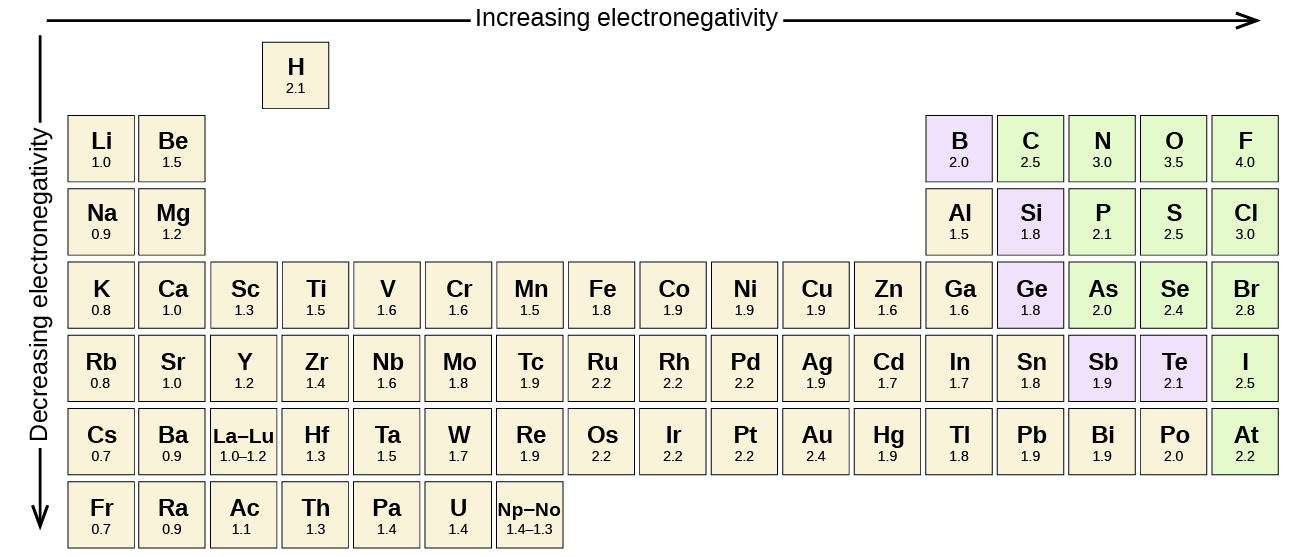
Across a period, the number of protons increases, therefore the positive charge on the nucleus increases and consequently the attraction for the outer electrons increases and the bonding pair of electrons will be attracted more strongly.
As you go down a group, the number of energy shells increases so a bonding pair of electrons will be increasingly further away from the nucleus. The electrons on the outer energy shell are further away from the nucleus and the bonding pair of electrons are attracted less strongly.
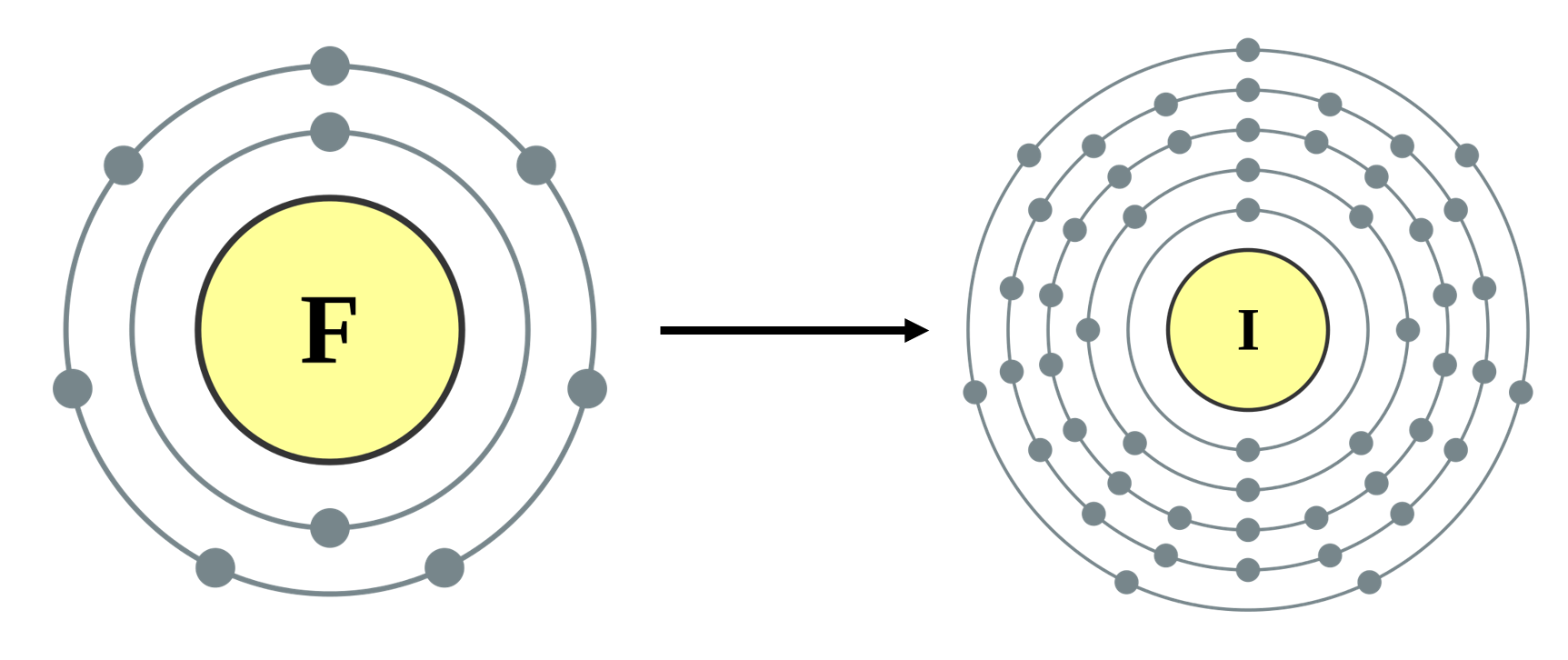
The greater the electronegativity of an atom of an element, the stronger its attractive pull on a bonding pair of electrons. For example, in a molecule of hydrogen bromide (HBr), the electronegativity of bromine [latex]\scriptsize (2.8)[/latex] is higher than that of hydrogen [latex]\scriptsize (2.1)[/latex], so the shared electrons will spend more of their time closer to the bromine atom. Bromine will have a slightly negative charge, and hydrogen will have a slightly positive charge. In a molecule like hydrogen (H2) where the electronegativities of the atoms in the molecule are the same, the bonding pair of electrons spend equal time around each atom so both atoms have a neutral charge.
Electronegativity and bonding
The electronegativity difference between two atoms can be used to determine what type of bonding exists between the atoms. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. So, it determines how the shared electrons are distributed between the two atoms in a bond. The higher the electronegativity, the more strongly an atom attracts the electrons in its bonds and consequently the bond formed is polar.
These are some general rules:
- If the electronegativity difference between two atoms in a bond is less than [latex]\scriptsize 1.7[/latex], then the bond is polar covalent.
- If the electronegativity difference between two atoms in a bond is greater than [latex]\scriptsize 2.0[/latex], then the bond is ionic.
- If the electronegativity difference between two atoms in a bond is between [latex]\scriptsize 1.6-\text{2}\text{.0}[/latex] and if a metal is involved, then the bond is considered ionic.
- If the electronegativity difference between the atoms is [latex]\scriptsize \displaystyle 0[/latex], the bond is pure covalent.
Exercise 2.1
Choose the correct answer for each question.
- Electronegativity is the ability of an atom to _____
- repel protons.
- repel electrons.
- attract neutrons.
- attract protons.
- attract electrons.
- As you move from left to right on the periodic table, what happens to the electronegativity?
- It decreases.
- It remains constant.
- It decreases, then increases with the noble gases.
- It increases.
- As you move from top to bottom on the periodic table, why does the electronegativity decrease?
- Because the outermost electrons get farther and farther away from the atom’s nucleus, the attraction between the nucleus and the bonding electrons decreases.
- Because the outermost electrons get closer and closer to the atom’s nucleus, the attraction between the nucleus and the bonding electrons decreases.
- Because the outermost electrons get farther and farther away from the atom’s nucleus, the attraction between the nucleus and the bonding electrons increases.
- Because the outermost electrons get closer and closer to the atom’s nucleus, the attraction between the nucleus and the bonding electrons increases.
The full solutions can be found at the end of the unit.
Polarity of molecules
is the property of being polar.
Molecules are described as being polar when there is an overall unequal distribution of charge creating one side with a positive charge and one side with a negative charge. This happens when the bonds between the atoms are polar, and the molecule has an asymmetrical shape.
Water is a polar molecule because of the polar bonds between the oxygen and the hydrogen atoms and its asymmetrical shape.
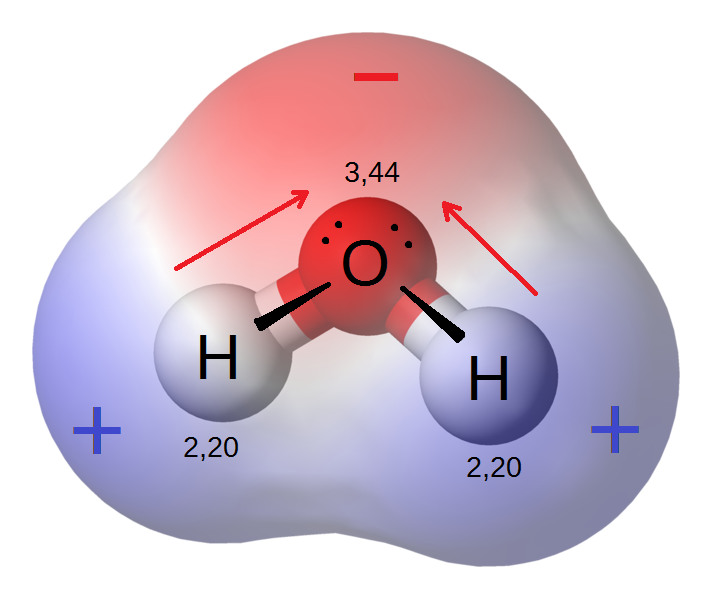
As you can see in figure 3, the water molecule is made up of oxygen and hydrogen, with respective electronegativities of [latex]\scriptsize 3.44[/latex] and [latex]\scriptsize 2.20[/latex]. The electronegativity difference polarises each H–O bond, shifting the electrons towards the oxygen. This gives the oxygen side of the molecule a negative charge and the side with the hydrogen atoms a positive charge.
Electrons in a polar covalent bond are shifted towards the more electronegative atom, giving this side of the molecule a partial negative charge. The greater the difference in electronegativity, the more polarised the electron distribution and the greater the polarity of the molecule.
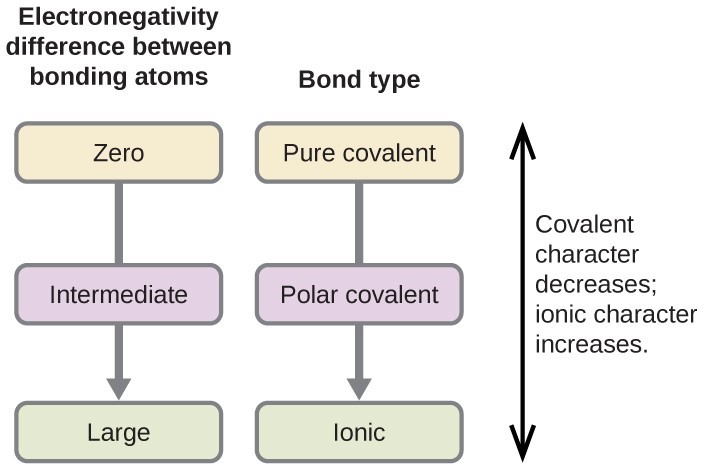
| Electronegativity difference | Type of bond |
| [latex]\scriptsize 0[/latex] | Pure covalent |
| [latex]\scriptsize 0-1[/latex] | Weak polar covalent |
| [latex]\scriptsize 1.1-1.9[/latex] | Strong polar covalent |
| [latex]\scriptsize \ge 2.0[/latex] | Ionic |
Remember that when the electronegativity difference is between [latex]\scriptsize 1.6-1.9[/latex] and a metal is involved in the formation of the compound, the type of bond will be ionic.
Let’s look at an example of how to calculate the electronegativity difference between molecules.
Example 2.1
Calculate the electronegativity difference between hydrogen and oxygen.
Solution
Read the electronegativity of each element off the periodic table.
From the periodic table we find that hydrogen has an electronegativity of [latex]\scriptsize 2.1[/latex] and oxygen has an electronegativity of [latex]\scriptsize 3.5[/latex]
Calculate the electronegativity difference
[latex]\scriptsize 3.5-2.1=1.4[/latex]
Chlorine is an example of non-polar covalent bond. The electronegativity difference is [latex]\scriptsize 0[/latex] because both atoms have an electronegativity value of [latex]\scriptsize 3.0[/latex], and [latex]\scriptsize 3.0-3.0=0[/latex].
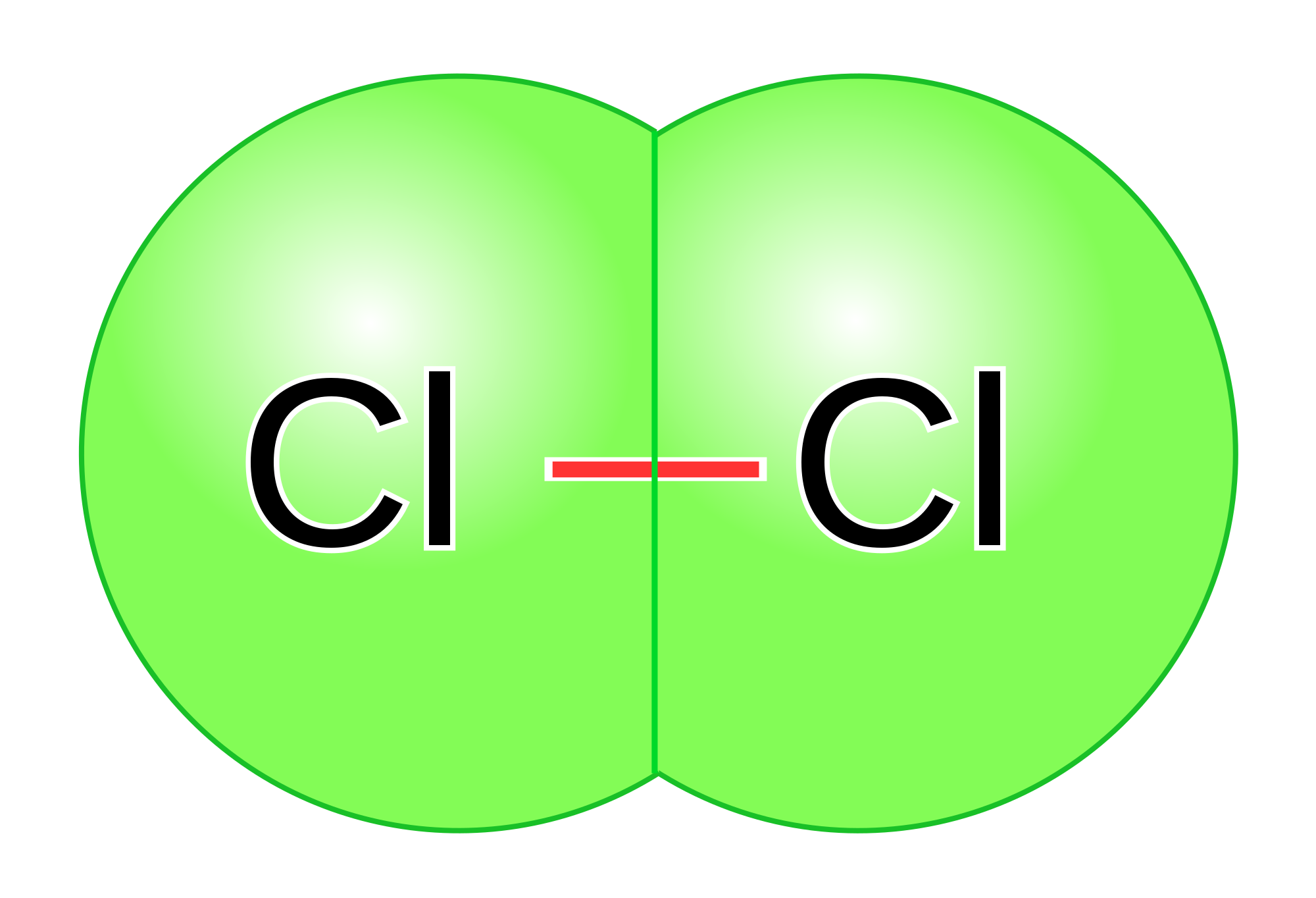
Because the bond is non-polar, there is no unequal distribution of charge across the molecule. Therefore the molecule will be non-polar. The polarity of the molecules in a substance affects its properties such as its boiling point, melting point, and solubility.
Summary
In this unit you have learnt the following:
- Electronegativity is a chemical property which describes the power of an atom to attract electrons towards itself.
- Electronegativity can be used to explain the difference between two types of covalent bonds: polar covalent bonds (between non-identical atoms) and non-polar/pure covalent bonds (between identical atoms or atoms with the same electronegativity).
- A polar molecule is one that has one end with a slightly positive charge, and one end with a slightly negative charge, examples include water, ammonia, and hydrogen chloride.
- A non-polar molecule is one where the charge is equally spread across the molecule.
Unit 2: Assessment
Suggested time to complete: 20 minutes
- Define electronegativity.
- .
- On the Pauling scale the electronegativities of nitrogen and oxygen are respectively [latex]\scriptsize \displaystyle 3.0[/latex] and [latex]\scriptsize \displaystyle 3.5.[/latex] Why is oxygen more electronegative than nitrogen?
- On the same scale, the electronegativity of sulphur is [latex]\scriptsize \displaystyle 2.5[/latex]. Why is sulphur less electronegative than oxygen?
- By thinking about where the following atoms are in the periodic table, sort them into order of increasing electronegativity:
aluminium
barium
boron
caesium
calcium
carbon
fluorine - Given the following:
oxygen (O2), magnesium chloride (MgCl2) sodium chloride (NaCl) and sulfuric acid (H2SO4)- Which substance/s has/have non-polar bonds? Explain your answer.
- Calculate the electronegativity difference for:
- Sodium and chlorine
- Magnesium and chlorine
The full solutions can be found at the end of the unit.
Unit 2: Solutions
Exercise 2.1
- e
- d
- a
Unit 2: Assessment
- Electronegativity is the ability of an atom to attract and hold onto a bonding pair of electrons.
- .
- Oxygen is more electronegative than nitrogen because it has more protons. The positively charged protons allow oxygen to have a stronger force of attraction on electrons.
- Sulfur is less electronegative than oxygen because it is in period [latex]\scriptsize \displaystyle 3[/latex]. This means that it has three energy shells compared to oxygen which has two. The electrons on the 3rd level are further away from the nucleus and the force of attraction will be weaker.
- .
Fluorine
Carbon
Boron
Aluminium
Calcium
Caesium
Barium - .
- Two oxygen atoms have an electronegative difference of [latex]\scriptsize \displaystyle 0[/latex]. (Sodium chloride and magnesium chloride are ionically bonded because both sodium and magnesium are metals.)
- .
- [latex]\scriptsize 3-0.9=2.1[/latex]
- [latex]\scriptsize 3-1.2=1.8[/latex]
Media Attributions
- Fig 1 © Rice University is licensed under a CC BY (Attribution) license
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
- Fig 3 © Riccardo Rovinetti is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 4 © Rice University is licensed under a CC BY (Attribution) license
- Fig 5 © すじにくシチュー is licensed under a CC0 (Creative Commons Zero) license
the chemical property which describes the power of an atom to attract bonding pairs of electrons towards itself
the unequal distribution of charge caused by the difference in electronegativity; this term can be applied to a bond or molecule
