Mechanical Properties of matter: State, analyse and explain change of state due to molecular forces
Unit 2: The effect of mass and intramolecular forces
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Understand and identify that intramolecular forces act within physical states of matter.
- Understand and identify the effect molecular mass has on boiling point.
- Understand and identify that the strength of both hydrogen bonds and van der Waals forces affect the boiling point of elements in groups [latex]\scriptsize \displaystyle 15-17[/latex].
What you should know
Before you start this unit, make sure you can:
- Understand intermolecular and intramolecular forces. Refer to level 3, subject outcome 5.3, unit 1 to revise this.
Introduction
The type of intermolecular force determines whether a substance is soluble in different solvents and affects its melting and boiling points. Molecular mass also affects a substance’s melting and boiling point. The higher the molecular mass the higher the boiling and melting points and vice versa. Hydrogen bonds and van de Waals forces will also affect the boiling point of elements in groups [latex]\scriptsize \displaystyle 15-17[/latex]. These groups are made up of mainly non-metals and the stronger the forces the higher the melting point.
Intramolecular forces
Intramolecular forces are the forces of attraction between atoms in a molecule. There are three different types of intramolecular forces: ionic, covalent, and metallic. These forces are responsible for the formation of chemical bonds. Thus, intramolecular forces are much stronger than intermolecular forces. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. When electrons are shared between two atoms, the bond is called a covalent bond. When one atom gives/gains electrons, the bond is called an ionic bond.
Intermolecular forces and boiling points
Intermolecular forces dictate several properties, such as melting points, boiling points, and solubilities of substances. Molar mass, molecular shape, and polarity affect the strength of different intermolecular forces, which influence the magnitude of physical properties across a family of molecules. Temporary attractive forces such as dispersion are present in all molecules, whether they are polar or nonpolar. They cause gases to condense (liquefy) and liquids to freeze (solidify) under very low temperature (or high pressure) conditions. Dispersion forces arise from temporary dipoles caused by the asymmetrical distribution of electrons around the atom’s nucleus. Atoms (or molecules) with a greater number of electrons (a higher molecular mass) display stronger dispersion forces than atoms (or molecules) with a lower molecular mass.
We know that raising the temperature of a substance will increase the kinetic energy of molecules, and if the kinetic energy is raised high enough, then the molecules will be able to overcome the electrostatic attraction of the intermolecular forces. If the molecules in a liquid can move apart, then the free molecules will form a gas, causing the liquid to boil.
Intermolecular forces can be used to predict boiling points. The stronger the intermolecular force, the higher the boiling point. Therefore, we can compare the relative strengths of the van der Waals forces of the compounds of similar molecular mass to predict their relative boiling points:
H-bonding > dipole-dipole > London dispersion
London dispersion forces
London dispersion forces are the weakest intermolecular force that arise from temporary dipole moments in nonpolar molecules. These temporary dipoles influence neighbouring nonpolar molecules and weak dipole-induced dipole interactions form. These forces cause nonpolar substances to condense into liquids and freeze to solids if the temperature is low enough.
Melting and boiling points increase as you move down the non-metal groups of the periodic table. These increase because of increases in . The strength of London dispersion forces are affected by the electronic structure of the atoms or molecules in the substance. In a larger atom, the are further from the nuclei than in a smaller atom; they are less tightly held and can more easily form the temporary dipoles that produce the attraction.
As dispersion forces become stronger, the electrostatic force of attraction between molecules becomes stronger and melting and boiling points of covalent substances thus increase with larger molecular mass.
As you move down the halogens group, from fluorine to iodine, melting points and boiling points increase with increasing atomic mass.
| Halogen | Molar mass (g/mol) | Melting point (0C) | Boiling point (0C) |
| Fluorine (F2) | [latex]\scriptsize 38[/latex] | [latex]\scriptsize -220[/latex] | [latex]\scriptsize -188[/latex] |
| Chlorine (Cl2) | [latex]\scriptsize 71[/latex] | [latex]\scriptsize -101[/latex] | [latex]\scriptsize -35[/latex] |
| Bromine (Br2) | [latex]\scriptsize 106[/latex] | [latex]\scriptsize -7[/latex] | [latex]\scriptsize 59[/latex] |
| Iodine (I2) | [latex]\scriptsize 254[/latex] | [latex]\scriptsize 114[/latex] | [latex]\scriptsize 184[/latex] |
| Astatine (At2) | [latex]\scriptsize 420[/latex] | [latex]\scriptsize 302[/latex] | [latex]\scriptsize 337[/latex] |
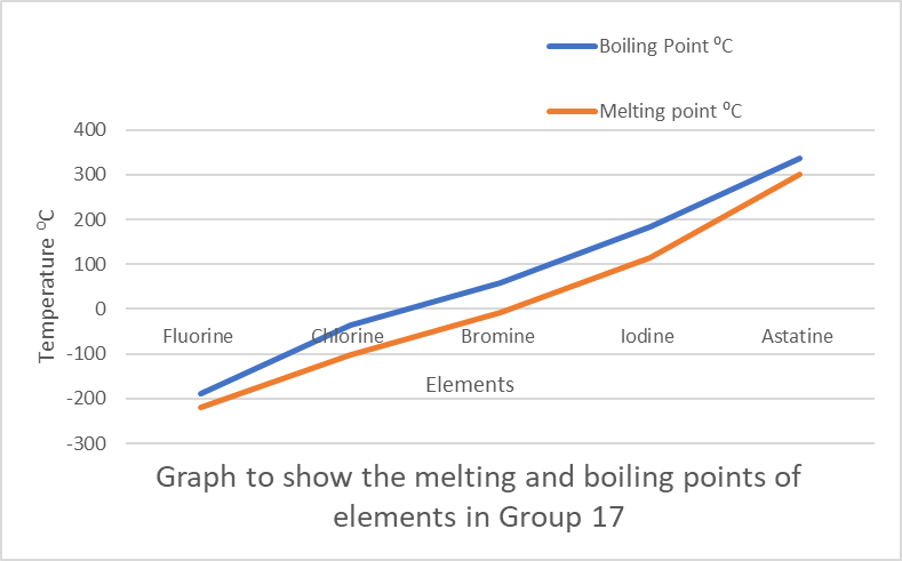
Exercise 2.1
You will need graph paper for this exercise, and you will need to use the information in Table 1 for the following questions.
- On a piece of graph paper, plot a graph of the mass vs boiling point of the halogens.
- Explain the trend on the graph.
The full solutions can be found at the end of the unit.
Polar substances exhibit dipole-dipole attractions. The effect of this attraction can be seen when comparing the properties of polar hydrogen chloride (HCl) molecules to nonpolar fluorine (F2) molecules. Both HCl and F2 consist of the same number of atoms and have approximately the same molecular mass. At a temperature of [latex]\scriptsize \displaystyle \text{-123}\text{.15}{{\text{ }}^{^\circ }}\text{C}[/latex], molecules of both substances would have the same average kinetic energy.
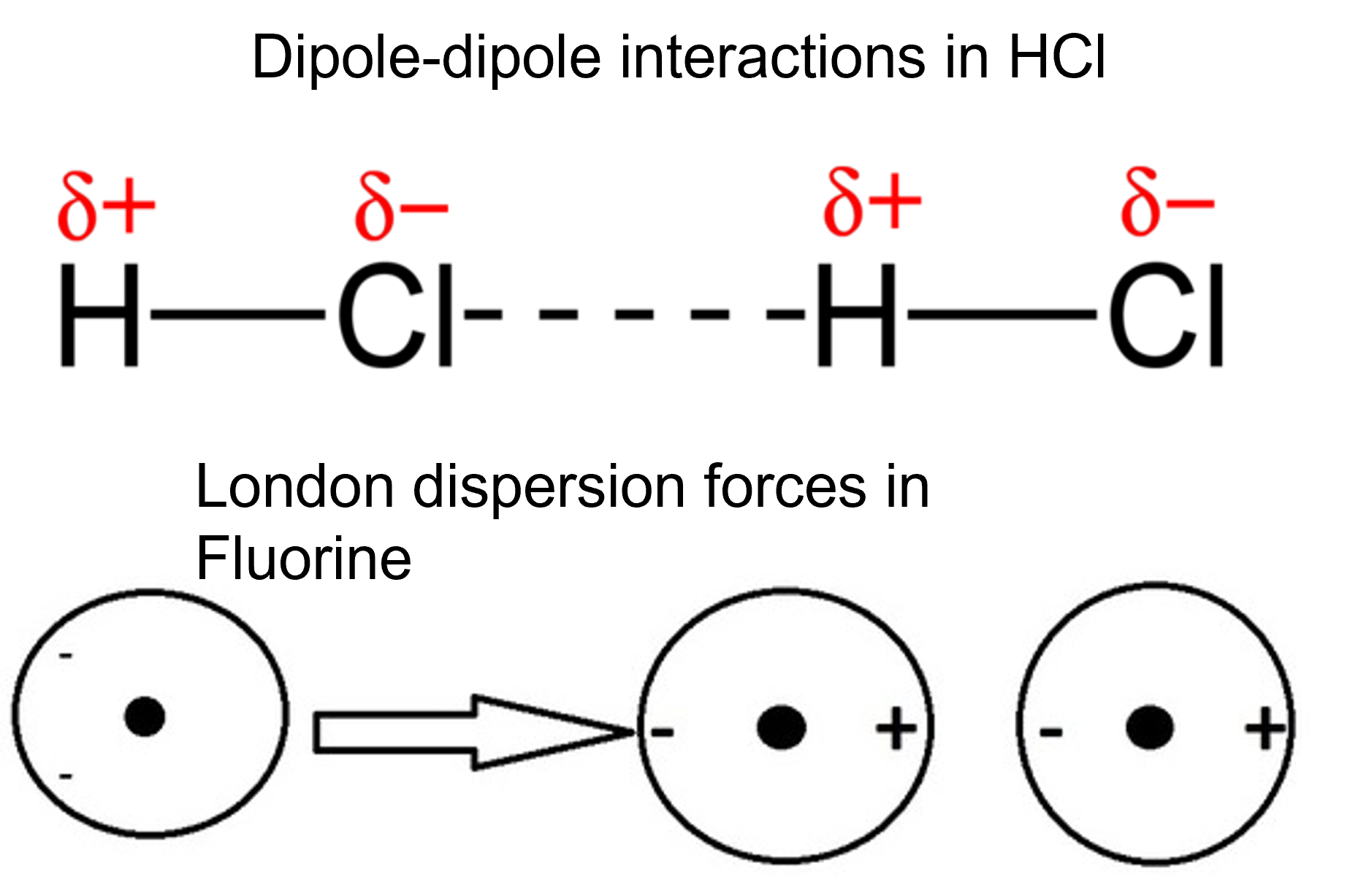
However, the dipole-dipole attractions between HCl molecules are sufficient to cause them to ‘stick together’ to form a liquid, whereas the relatively weaker London dispersion forces between nonpolar Fluorine molecules are not, and so this substance is gaseous at this temperature. The higher normal boiling point of HCl at [latex]\scriptsize -85{{\text{ }}^{0}}\text{C}[/latex] compared to F2 at [latex]\scriptsize -188{{\text{ }}^{\text{0}}}\text{C}[/latex] reflects the greater strength of dipole-dipole attractions between HCl molecules, compared to the attractions between nonpolar F2 molecules.
Hydrogen bonds and hydrides
The presence of hydrogen bonds has a significant effect on the physical properties of substances in which they are found.
Hydrogen can form compounds with less electronegative elements and are known as . So when hydrogen reacts with any other element the product formed is a hydride.
A hydrogen molecule usually reacts with many elements except noble gases to form hydrides. However, the properties of these compounds may vary depending on the type of intermolecular force that exists between the molecules, its molecular mass, temperature, and other factors.
Covalent hydrides are formed when hydrogen reacts with other elements with similar electronegativity like Si, C, and sulfur. The most common examples are methane and hydrogen sulfide. In general, compounds that are formed when hydrogen is reacted with non-metals are called . Covalent hydrides can be either liquids or gases at room temperature and can be or non-volatile.
Not all compounds with hydrogen have hydrogen bonding. Let us look at two different chemicals with a similar molecular geometry but different types of intermolecular forces: water and hydrogen sulfide (H2S). Even though H2S has a higher molecular mass than water, water has hydrogen bonding and hydrogen sulfide has dipole-dipole forces. The boiling point of hydrogen sulfide ([latex]\scriptsize -60{{\text{ }}^{\text{0}}}\text{C}[/latex]) is far lower than the boiling point of water ([latex]\scriptsize 100{{\text{ }}^{0}}\text{C}[/latex]). Hydrogen sulfide does not have hydrogen bonding since sulfur has a low electronegativity. This reduces the boiling point of hydrogen sulfide because less energy is required to overcome the dipole-dipole forces between molecules of hydrogen sulfide than the hydrogen bonds of water.
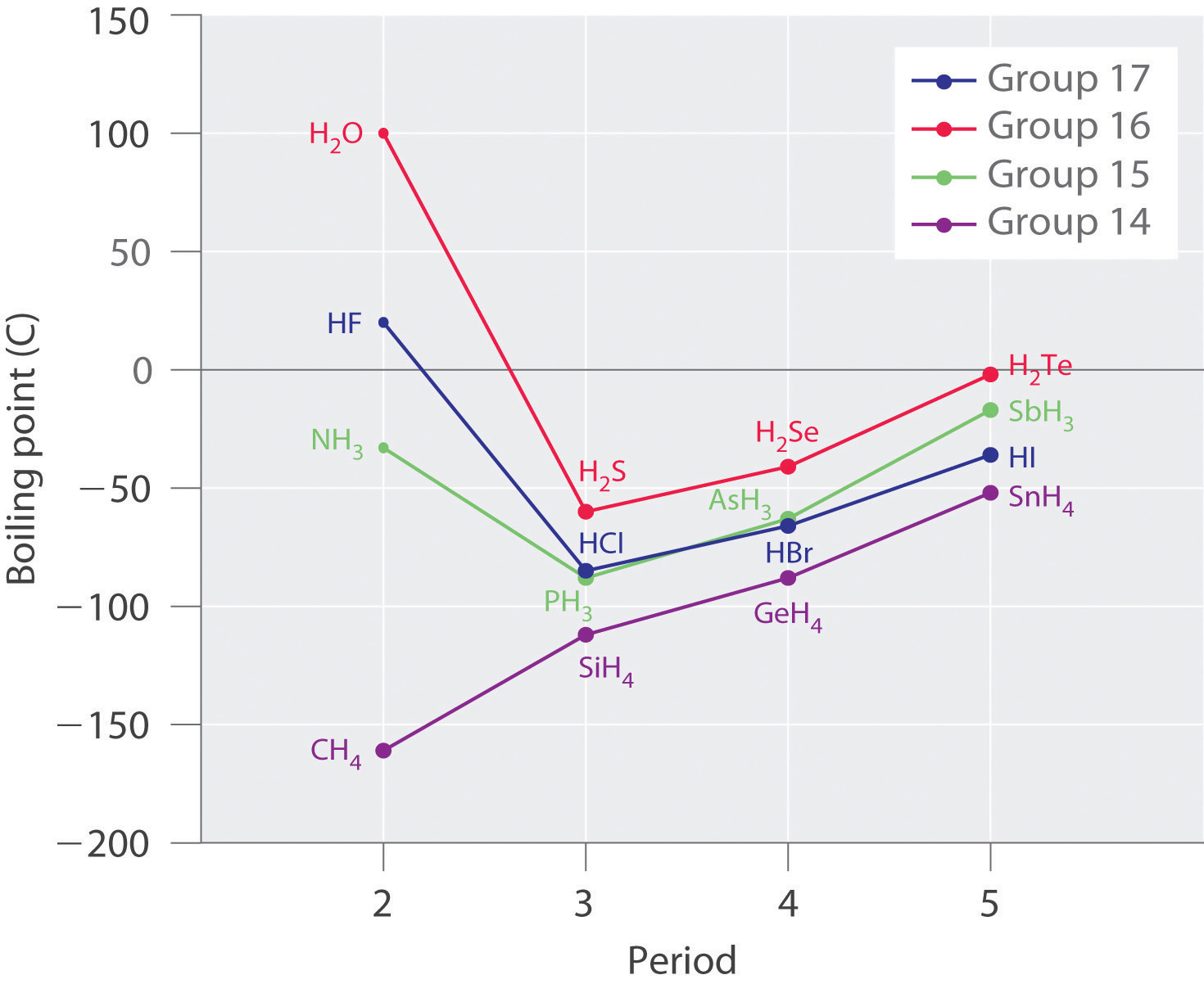
If we consider the general trend in boiling points for the hydrides of group [latex]\scriptsize \displaystyle 15[/latex], group [latex]\scriptsize \displaystyle 16[/latex] and group [latex]\scriptsize \displaystyle 17[/latex] in the graph in figure 3, it is clear that the effect of increasingly stronger dispersion forces dominates that of increasingly weaker dipole-dipole attractions, and the boiling points increase steadily with increasing molecular mass. The much higher than expected boiling points for ammonia, water and hydrogen fluoride than their molecular size would indicate are a result of them having hydrogen bonding. The measured boiling points for these compounds are about [latex]\scriptsize \displaystyle -33.34\text{ }^\circ \text{C}[/latex] for NH3, [latex]\scriptsize \displaystyle 100\text{ }^\circ \text{C}[/latex] for H2O, and [latex]\scriptsize \displaystyle 19.5\text{ }^\circ \text{C}[/latex] for HF. These boiling points highlight the strength of hydrogen bonding.
Summary
In this unit you have learnt the following:
- Hydrogen bonds are a type of dipole-dipole force that occurs when a hydrogen atom is bonded to a highly electronegative atom (oxygen, fluorine, nitrogen). The hydrogen atom on one molecule is attracted to the electronegative atom on a neighbouring molecule.
- Intermolecular forces affect the physical properties of substances.
- Substances with larger molecules have stronger intermolecular forces than substances with smaller molecules.
- Substances with weak intermolecular forces will have low melting and boiling points while those with strong intermolecular forces will have high melting and boiling points.
- Hydrides are hydrogen compounds formed with elements with a lower electronegativity.
- Hydrogen forms covalently bonded compounds with elements in groups [latex]\scriptsize \displaystyle 15-17[/latex].
- Hydrogen bonding between elements will cause the compound to have a higher than expected melting and boiling point.
Unit 2: Assessment
Suggested time to complete: 25 minutes
- Refer to the list of substances below:
.
HCl, Cl2, H2O, NH3, N2, HF
.
Select the most correct statement from the list below:- [latex]\scriptsize \displaystyle \text{N}{{\text{H}}_{\text{3}}}[/latex] is a non-polar molecule.
- The melting point of [latex]\scriptsize \displaystyle \text{N}{{\text{H}}_{\text{3}}}[/latex] will be higher than for [latex]\scriptsize \displaystyle \text{C}{{\text{l}}_{\text{2}}}[/latex].
- Ion-dipole forces exist between molecules of HF.
- At room temperature [latex]\scriptsize \displaystyle {{\text{N}}_{\text{2}}}[/latex] is usually a liquid.
- The following table gives the melting points of various hydrides:
Hydride Melting point (0C) Hydrogen iodide (HI) [latex]\scriptsize -34[/latex] Ammonia (NH3) [latex]\scriptsize -33[/latex] Hydrogen sulfide (H2S) [latex]\scriptsize -60[/latex] Methane (CH4) [latex]\scriptsize \displaystyle -164[/latex] - In which of these hydrides does hydrogen bonding occur?
- HI only
- [latex]\scriptsize \displaystyle \text{N}{{\text{H}}_{\text{3}}}[/latex] only
- HI and [latex]\scriptsize \displaystyle \text{N}{{\text{H}}_{\text{3}}}[/latex] only
- HI, [latex]\scriptsize \displaystyle \text{N}{{\text{H}}_{\text{3}}}[/latex] and [latex]\scriptsize \displaystyle {{\text{H}}_{\text{2}}}\text{S}[/latex]
- Draw a graph to show the melting point of these hydrides.
- In which of these hydrides does hydrogen bonding occur?
- The respective boiling points for four chemical substances are given below:
Hydride Melting point (0C) Hydrogen sulfide (H2S) [latex]\scriptsize -60[/latex] Ammonia (NH3) [latex]\scriptsize -33[/latex] Hydrogen fluoride (HF) [latex]\scriptsize 20[/latex] Water (H2O) [latex]\scriptsize 100[/latex] - Which substance exhibits the strongest forces of attraction between its molecules in the liquid state?
- Give the name of the force responsible for the relatively high boiling points of hydrogen fluoride and water.
- The shapes of the molecules of hydrogen sulfide and water are similar, yet their boiling points differ. Explain.
The full solutions can be found at the end of the unit.
Unit 2: Solutions
Exercise 2.1
- .
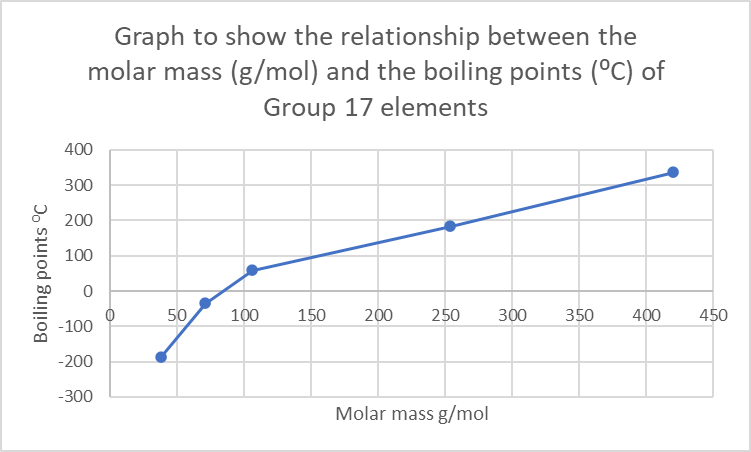
- As the mass of the element increases, the boiling point increases. This is because the strength of the intermolecular forces increases.
Unit 2: Assessment
- b. (The melting point of NH3 will be higher than for Cl2.).
- .
- ii. [latex]\scriptsize \displaystyle \text{N}{{\text{H}}_{3}}[/latex] only
- .
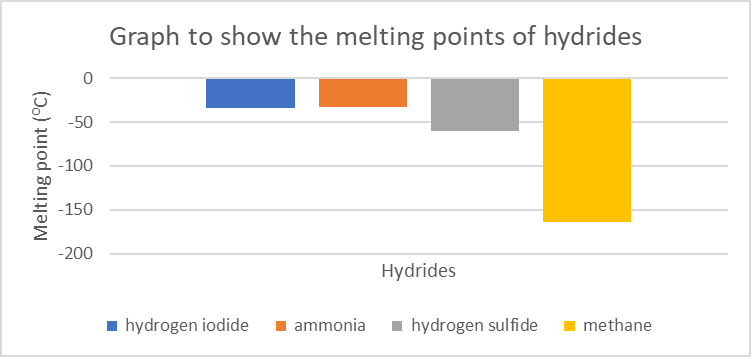
- .
- water
- hydrogen bonding
- Hydrogen sulfide does not have hydrogen bonding since sulfur has a low electronegativity. This reduces the boiling point of hydrogen sulfide since it requires less energy to overcome the weaker dipole-dipole intermolecular forces between molecules of hydrogen sulfide than the hydrogen bonding in water.
Media Attributions
- Fig 1 © DHET is licensed under a CC BY (Attribution) license
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
- Fig 3 © Libretext is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 4 © DHET is licensed under a CC BY (Attribution) license
- Fig 5 © DHET is licensed under a CC BY (Attribution) license
the average mass of an atom of an element, calculated using the relative abundance of all isotopes in a naturally occurring element
the number of electrons on the outermost energy level
any compound of hydrogen with another element
compounds in which hydrogen is covalently attached to a less electronegative element
likely to change suddenly and unexpectedly, especially by getting worse
