Mechanical Properties of matter: State, analyse and apply principles of atomic combinations: molecular structure
Unit 4: Molecular shape
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
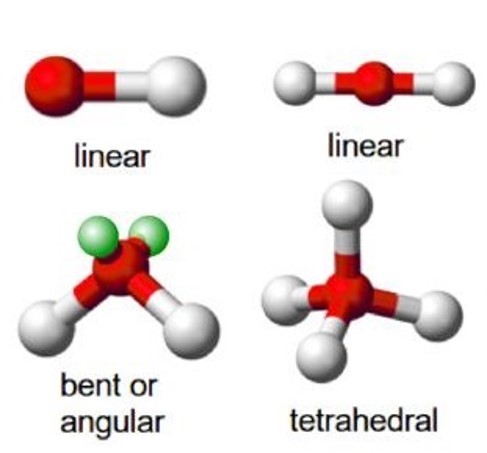
- Identify molecular shapes and give molecular examples for the shapes. Range: Molecular shapes are linear, angular, pyramidal, tetrahedral.
What you should know
Before you start this unit, make sure you can:
- Understand molecular structure. Refer to level 3 subject outcome 5.2 unit 1 to revise this.
- Understand electronegativity and polarity of molecules. Refer to level 3 subject outcome 5.2 unit 2 to revise this.
Introduction
Parts of the text in this unit were sourced from Siyavula Physical Science Gr 11 Learner’s Book, Chapter 3, released under a CC-BY licence.
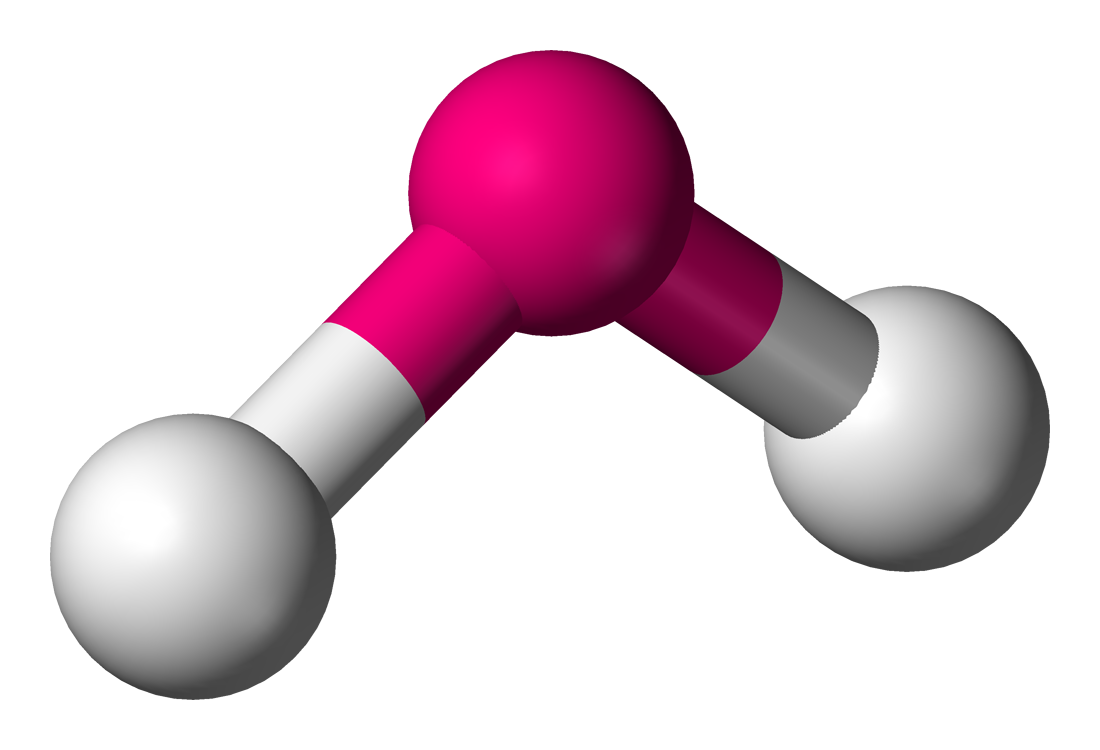
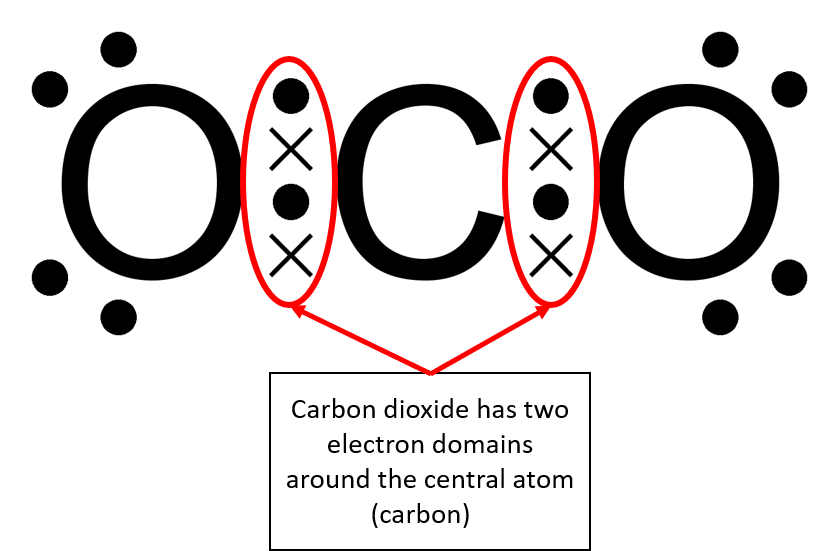
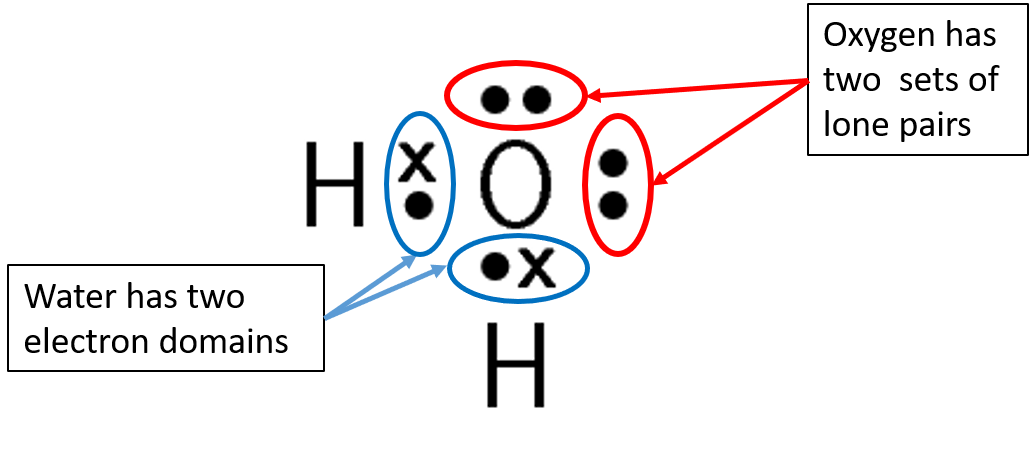
In this unit you will learn about molecular shape and give molecular examples for these shapes. The shape of a molecule is determined by electron pairs which repel each other to get as far away as possible from each other. Carbon dioxide will form a linear shape because the electron pairs on the oxygen atoms are trying to get as far away from each other as possible. Water forms an angular molecule because of the two electron
Molecular shape
(the shape that a single molecule has) is important in determining how the molecule interacts with other molecules around it. Molecular shape also influences the boiling point and melting point of the molecule. If all molecules were linear then life as we know it would not exist. Many of the varying properties of molecules are because of the particular shape that a molecule has. For example, if the water molecule were , it would be and so would not have all the special properties it has that make life possible on earth.
The of molecules are determined by the number of lone pairs and bonding pairs on the central atom. The total number of electron pairs, both and , lead to what is called the . This determines the 3-D shape of the molecule.
Electron domain geometry
You know that when you hold two like poles of a magnet close to each other they will repel each other. Lone pairs of electrons repel each other in the same way.

An atom’s electron domain is the number of lone pairs or chemical bond locations that surround it. It represents the number of locations expected to contain electrons. By knowing the electron domain of each atom in a molecule, you can predict its geometry. This is because electrons distribute around an atom to minimise repulsion with one another.
Electron repulsion is not the only factor that affects molecular geometry. Electrons are attracted to positively charged nuclei. The nuclei, in turn, repel each other. Electrons repel one another, so when they are placed near one another, they automatically organise themselves into a shape that minimises repulsions among them. This phenomenon is described as , or valence shell electron pair repulsion.
Valence shell electron pair repulsion (VSEPR) theory
The shape of a covalent molecule can be predicted using the valence shell electron pair repulsion (VSEPR) theory. Very simply, VSEPR theory says that the valence electron pairs in a molecule will arrange themselves around the central atom(s) of the molecule so that the repulsion between their negative charges is as small as possible. In other words, the valence electron pairs arrange themselves so that they are as far apart as they can be.
The central atom is the atom around which the other atoms are arranged. In a molecule of water, the central atom is oxygen. In a molecule of ammonia, the central atom is nitrogen.
VSEPR theory is based on the idea that the geometry (shape) of a molecule is mostly determined by repulsion among the pairs of electrons around a central atom. The pairs of electrons may be bonding or lone pairs. Only valence electrons of the central atom influence the molecular shape in a meaningful way. Electrons generally try to maximise distance from each other, but they are influenced by other forces, such as the proximity and size of a positively charged nucleus.
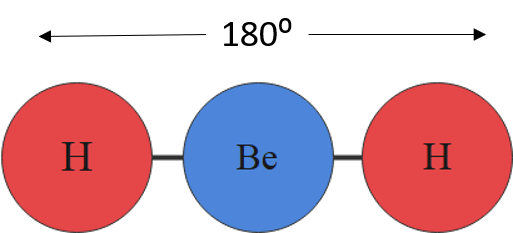
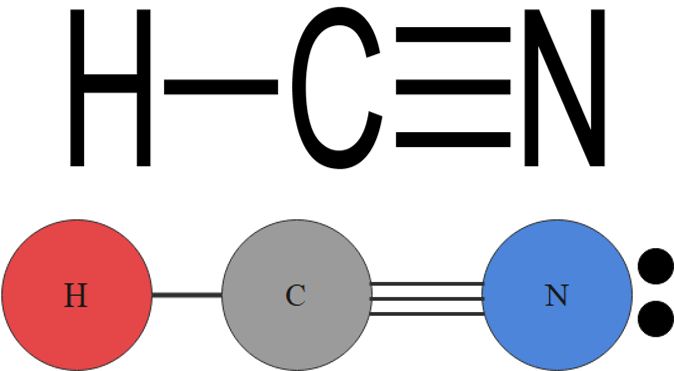
For example, a molecule with two bonding pairs and no lone pairs around the central atom has a linear shape. Beryllium hydride, pictured in figure 5, carbon dioxide and hydrogen cyanide, pictured in figure 6, are examples of a linear molecules.
A molecule with four electron pairs and no lone pairs around the central atom would have a tetrahedral shape (see figure 7).
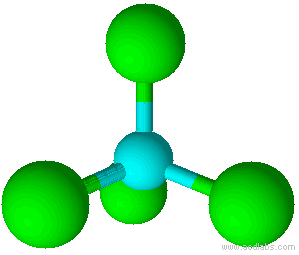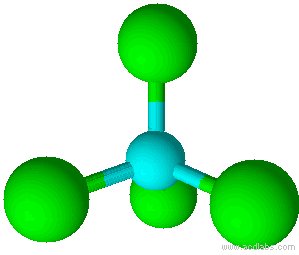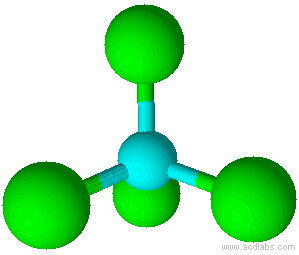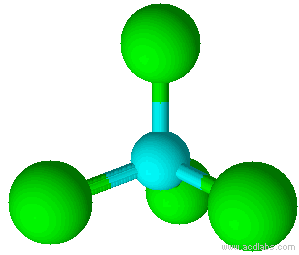
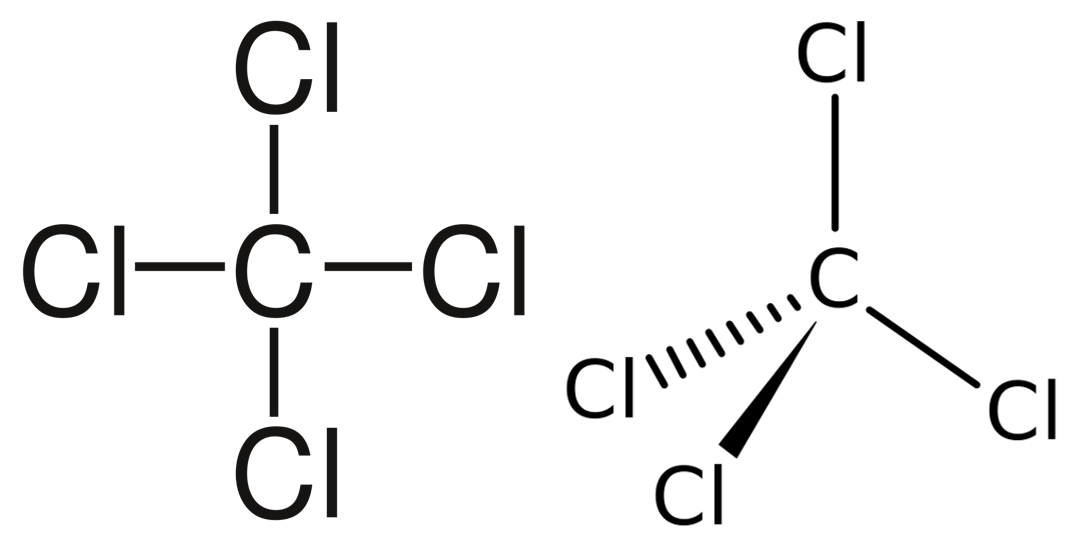
When there is a combination of bonding pairs and lone pairs around a central atom, the geometry gets distorted because of the varying strength of repulsion between these pairs.
The ammonia molecule contains three single bonds and one lone pair around one central nitrogen atom, so it has four electron domains around the central atom. The repulsion between the bonding pairs of electrons is not as strong as the repulsion between the lone pair and a bonding pair. This will distort the shape and the bond angle between the hydrogen atoms will decrease.
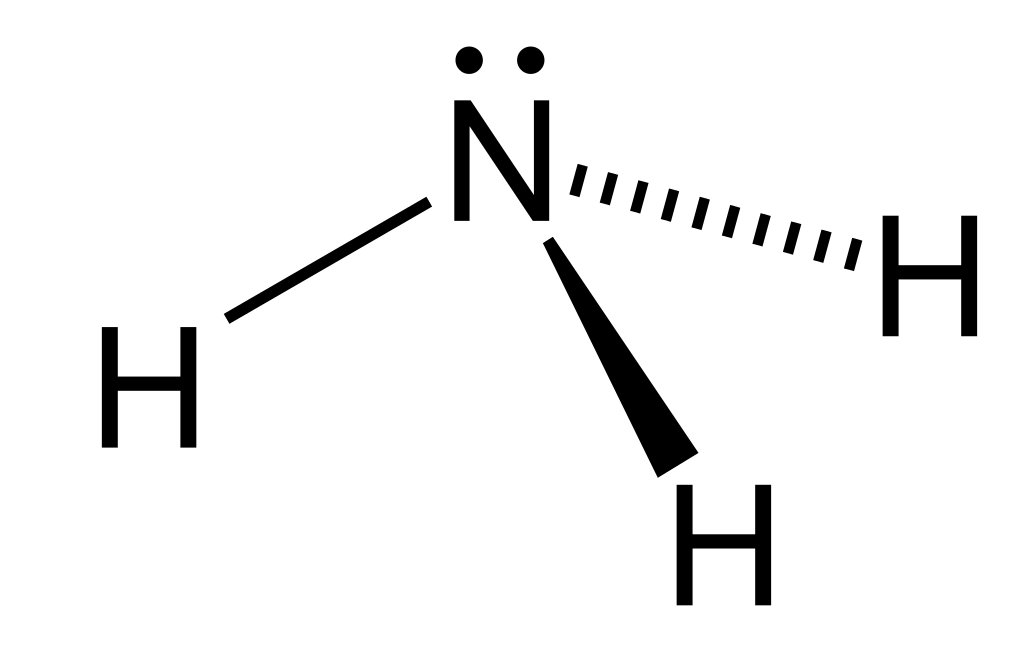
VSEPR theory predicts these distortions by establishing an order of strength of repulsions and the amount of space occupied by different kinds of electron pairs. The order of electron-pair repulsions from greatest to least repulsion is:
lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair
VSEPR theory predicts the arrangement of electron pairs around each central atom and thus the correct arrangement of atoms in a molecule. We should understand, however, that the theory only considers electron-pair repulsions. Other interactions, such as nuclear-nuclear repulsions and nuclear-electron attractions, are also involved in the final arrangement that atoms adopt in a particular molecular structure.
Determining molecular shape
- To predict the shape of a covalent molecule, follow these steps:
- Draw the molecule using a Lewis diagram. Make sure that you draw all the valence electrons around the molecule’s central atom.
- Count the number of electron pairs around the central atom.
Determine the basic geometry of the molecule using the table below.
Table 1 gives the common molecular shapes. In this table we use A to represent the central atom, X to represent the terminal atoms (i.e. the atoms around the central atom) and E to represent any lone pairs.
| Number of bonding electron pairs | Number of lone pairs | Geometry | Generic formula | Common examples |
| [latex]\scriptsize 1\text{ or }2[/latex] | [latex]\scriptsize 0[/latex] | linear | AX or AX2 | CO2 |
| [latex]\scriptsize 2[/latex] | [latex]\scriptsize 2[/latex] | bent or angular | AX2E2 | H2O |
| [latex]\scriptsize 4[/latex] | [latex]\scriptsize 0[/latex] | tetrahedral | AX4 | SF4 |
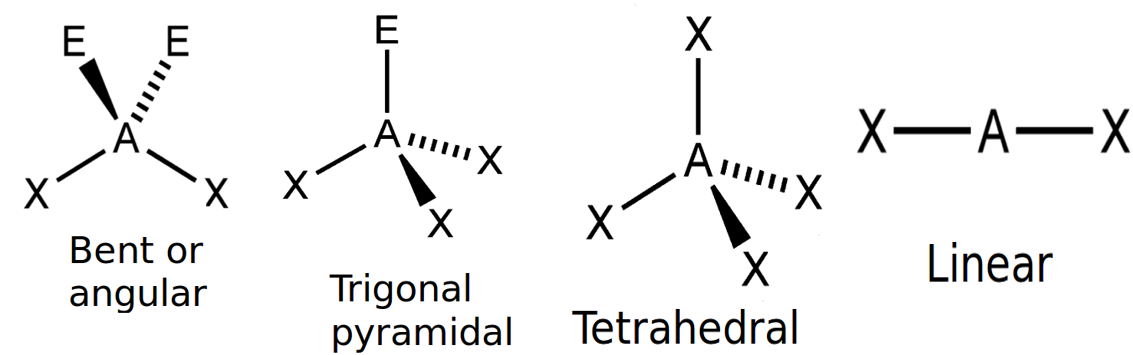
Of these shapes, the ones with no lone pairs are called the ideal shapes. The two ideal shapes are: linear and tetrahedral.
One important point to note about molecular shape is that all diatomic molecules (two identical atoms in a molecule) and molecules with only two atoms are linear. For example H2, HCl and Cl2 are all linear.
Example 4.1
What is the molecular shape of Chlorine?
Solution
Step 1: Draw the molecule using a Lewis diagram
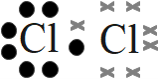
Step 2: Count the number of electron pairs around the atom
There is one electron pair.
Step 3: Determine the basic geometry of the molecule
There is one electron pair and no lone pairs. Cl2 has the general formula: AX2. Using this information and table 1 we find that the molecular shape is linear.
The molecular shape of Cl2 is linear.
Example 4.2
What is the molecular shape of Ammonia?
Solution
Step 1: Draw the molecule using a Lewis diagram
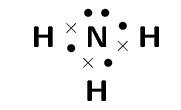
Step 2. Count the number of electron pairs around the central atom
There are four electron pairs.
Step 3. Determine the basic geometry of the molecule
There are three bonding electron pairs and one lone pair. The molecule has the general formula AX3E. Using this information and table 1 we find that the molecular shape is trigonal pyramidal.
The molecular shape of NH3 is trigonal pyramidal.
Activity 4.1: Build molecular models
Time required: 20 minutes
What you need:
- jelly tots or any soft sweets which come in different colours
- toothpicks
What to do:
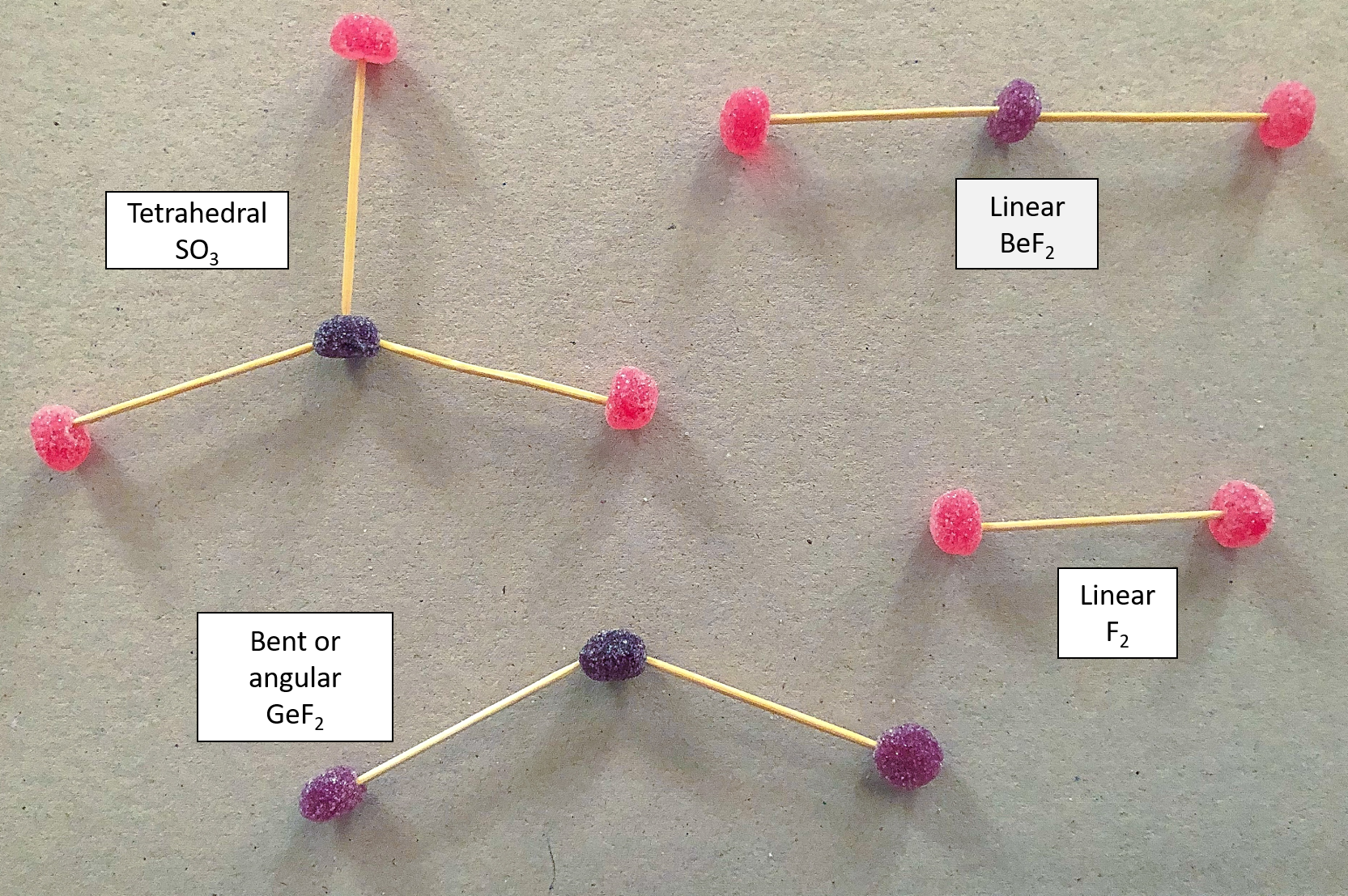
- Using two differently coloured jelly tots, build a model of CO2.
- What shape is the molecule?
- In your notebook, draw a Lewis structure and write down the shape.
- Repeat step 1 for the following molecules, remembering to use different coloured jelly tots to represent the different elements:
- F2
- BeF2
- SO3
- GeF2
Remember to use two toothpicks to represent a double bond and use table 1 to help you.
What did you find?
This is what your molecules should look like:
Summary
In this unit you have learnt the following:
- Valence shell electron pair repulsion (VSEPR) theory is a model in chemistry, which is used to predict the shape of individual molecules. VSEPR is based upon minimising the extent of the electron pair repulsion around the central atom being considered.
- Electrons repel one another; hence, the most stable arrangement of a given number of electron pairs is the one that minimises the repulsions among them.
- There are two types of valence shell electron pairs: bonding pairs and lone pairs.
- It is common to represent bonding patterns by generic formulas such as AX4, Ax2E2, etc., in which ‘X’ stands for bonding pairs and ‘E’ denotes lone pairs. This convention is known as the ‘AXE Method’.
Unit 4: Assessment
Suggested time to complete: 30 minutes
- Which one of the following is a linear molecule?
- HF
- BF3
- CH4
- CCl4
- The shape of carbon dioxide is described as:
- linear
- angular
- tetrahedral
- trigonal planar
- The molecule whose shape is based on lone pairs is:
- CH4
- CO2
- H2O
- SF6
- Which of the following statements about H2S molecules is true?
- The -H-S bond angle is [latex]\scriptsize \displaystyle 180^\circ[/latex].
- Their shape is based on them having two lone pairs and two double bond pairs.
- Their shape is based on them having one lone pair and two bonding pairs.
- They are tetrahedral.
- What are lone pairs? Explain how lone pairs affect molecular geometry.
- Determine the shape of the following molecules:
- H2O
- CH4
- C2H4
The full solutions can be found at the end of the unit.
Unit 4: Solutions
Unit 4: Assessment
- a
- a
- c
- c
- Lone pairs are two electrons which are not involved in the bonding in a molecule. They will repel the electrons in the bonding pair, causing the shape of a molecule to change.
- .
- angular
- tetrahedral
- linear
Media Attributions
- Fig 1 © Benjah-bmm27 is licensed under a CC0 (Creative Commons Zero) license
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
- Fig 3 © DHET is licensed under a CC BY (Attribution) license
- Fig 4 © DHET is licensed under a CC BY (Attribution) license
- Fig 5 © DHET is licensed under a CC BY (Attribution) license
- Fig 6 © DHET is licensed under a CC BY (Attribution) license
- Fig 7 © Libre Text is licensed under a CC BY-NC (Attribution NonCommercial) license
- Fig 8 © DHET is licensed under a CC BY (Attribution) license
- Fig 9 © Radio89 is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 10 © DHET is licensed under a CC BY (Attribution) license
- Fig 11 © DHET is licensed under a CC BY (Attribution) license
- Fig 12 © DHET is licensed under a CC BY (Attribution) license
- Fig 13 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 14 © DHET is licensed under a CC BY (Attribution) license
the shape of a molecule
arranged in a straight line (180 degrees)
where the charge is equally spread across the molecule or a symmetrical molecule with polar bonds
the arrangements in space of the atoms of a molecule
the electrons (one from each atom) involved in bonding
electron pairs on the outermost energy level which are not involved in bonding
bonding and lone pairs which determine the shape of a molecule
the theory based on minimising the repulsion around the central atom
