Mechanical Properties of matter: Describe, analyse and apply properties of gases theory
Unit 1: Gas theory
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Describe and identify properties of gases.
- Describe and identify kinetic-molecular theory.
- Describe and identify an ideal gas.
What you should know
Before you start this unit, make sure you can:
- Understand kinetic theory. Refer to level 2 subject outcome 5.1 unit 1 to revise this.
- Understand intermolecular forces. Refer to level 3 subject outcome 5.3 unit 1 to revise this.
Introduction
The particles in a gas have high kinetic energy but there are still weak forces of attraction between the particles. An ideal gas is defined as a theoretical gas whose particles have no force of attraction between them and all particles move at the same speed. We use the ideal gas theory to predict how gases behave within temperature and pressure ranges.
Kinetic theory of gases
The kinetic theory of matter, covered in level 2 subject outcome 5.1 unit 1, says that all matter is composed of particles that have a certain amount of energy which allows them to move at different speeds depending on the temperature. The temperature of a gas is a measure of the average kinetic energy of the particles. There are spaces between the particles and attractive forces between particles when they come close together.
If the gas is heated the average kinetic energy of the gas particles will increase and if the temperature is decreased, the average kinetic energy of the particles decreases. If the energy of the particles decreases significantly, the gas becomes a liquid.
The main assumptions of the kinetic theory of gases are as follows:
- Gases are made up of particles. The size of these particles is small compared to the distance between the particles and the total volume occupied by gas molecules themselves is negligible relative to the total volume of their container.
- Particles are constantly moving because they have kinetic energy. The particles move in straight lines at different speeds.
- There are weak attractive forces between particles.
- The collisions between particles and the walls of the container do not change the kinetic energy of the system.
- The average kinetic energy of gas particles is proportional to the absolute temperature of the gas, and all gases at the same temperature have the same average kinetic energy.
From these assumptions we can define the pressure and temperature of any gas.
An is a hypothetical gas that does not really exist in the environment. The concept of ideal gas was introduced since the behaviour of real gases is complicated and the behaviour of a real gas can be described with respect to the properties of an ideal gas. It is a useful starting point to study the properties of gases.
One of the assumptions of the kinetic theory of gases is that all particles have a different speed. However, this is only the case for a real gas. For an ideal gas we assume that all particles in the gas have the same speed. So for an ideal gas we can simply talk about the speed of particles.
But for a real gas we must use the average speed of all the particles. Real gases behave like ideal gases except at high pressures and low temperatures.
The pressure of a gas is a measure of the number of collisions of the gas particles with the sides of the container that they are in, per unit time.
Real gas behaviour
Real gas molecules have weak van der Waals intermolecular attractions between them. When two real gas particles collide with each other, a change in the energy of the particle and a change in the direction of its movement can be observed.
Real gases may behave as ideal gases under low pressure and high temperature conditions. At high temperatures, the kinetic energy of gas molecules is increased. Therefore the speed of gas molecules increases. This results in no intermolecular interactions between real gas molecules. At low pressures the particles are so far apart there will be no intermolecular interactions between them.
Ideal gases and non-ideal gas behaviour
Ideal gases are gaseous compounds that are composed of very tiny molecules that have a negligible volume and mass. As we already know, all real gases are composed of atoms or molecules that have a definite volume and mass. Ideal gases have no intermolecular forces between them. The atoms or molecules in an ideal gas move at the same speed. The collisions between ideal gas molecules are elastic. This means, there are no changes in the kinetic energy or the direction of the movement of the gas particles.
There are no attraction forces between ideal gas particles at any temperature or pressure. Therefore, particles move here and there freely, and consistently.
Almost all gases obey the ideal gas laws within a limited range of pressures and temperatures. It is only at low temperature and high pressures that real gases will behave differently from ideal gases:
-
Molecules of real gases do occupy volume.
When pressures are very high and the molecules are compressed, the volume of the molecules becomes significant. This means that the total volume available for the gas molecules to move is reduced and collisions become more frequent. This causes the pressure of the gas to be higher than what would be expected for an ideal gas.
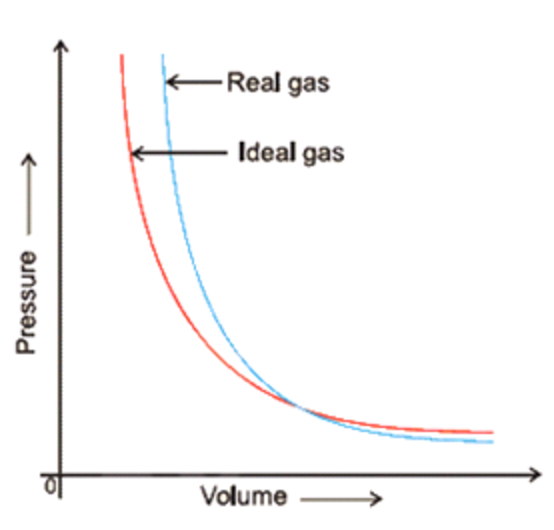
Figure 2: Real gases deviate from ideal gases at high pressure -
Forces of attraction do exist between molecules of real gases.
At low temperatures, when the speed of the molecules decreases and they move closer together, the intermolecular forces become more apparent. As the attraction between molecules increases, their movement decreases and there are fewer collisions between them. The pressure of the gas at low temperatures is therefore lower than what would have been expected for an ideal gas. If the temperature is low enough or the pressure high enough, a real gas will liquefy.
The differences between a real gas and an ideal gas
Here is a summary of the differences between a real gas and an ideal gas.
| Real gas | Ideal gas |
| Gaseous substance that really exists | Hypothetical gas which does not really exist |
| Intermolecular forces of attraction between the particles | No intermolecular forces of attraction between the particles |
| Have a definite volume and mass | Have no mass and no definite volume |
| Collisions between the particles are non-elastic | Collisions between the particles are elastic |
| The kinetic energy of the particles changes when there is a collision | The kinetic energy is constant |
| Particles may behave as an ideal gas when the temperature is high, and the pressure is low | May behave as a real gas when the temperature is low, and the pressure is high |
Summary
In this unit you have learnt the following:
- The kinetic theory of gases helps to explain the behaviour of gases under different conditions.
- The kinetic theory of gases states that gases are made up of constantly moving particles that have attractive forces between them.
- The pressure of a gas is a measure of the number of collisions of the gas particles with the sides of the container that they are in, per unit time.
- The temperature of a substance is a measure of the average kinetic energy of the particles.
- An ideal gas has identical particles of zero volume, with no intermolecular forces between the particles. The atoms or molecules in an ideal gas move at the same speed.
- A real gas behaves like an ideal gas, except at high pressures and low temperatures. At low temperatures, the forces between molecules become significant and the gas will liquefy. At high pressures, the volume of the particles becomes significant.
Unit 1: Assessment
Suggested time to complete: 10 minutes
For the following statements answer only with true or false:
- The conditions for a gas to behave ideally are high temperature and low pressure.
- As the temperature of gas particles increases, the average kinetic energy increases, and the speed of particles decreases.
- When ideal gas particles collide with something they lose some of their energy as heat.
- When real gas particles collide, they lose some energy as heat.
- An ideal gas does not exist.
- An ideal gas behaves like a real gas at high pressure and low temperature.
- An ideal gas has van der Waals forces of attraction between its particles.
- Real gas particles have constant kinetic energy and non-elastic movement.
- At a high temperature and low pressure a gas will change to a liquid.
- If a gas is compressed the pressure will increase as there are more collisions between the particles.
The full solutions can be found at the end of the unit.
Unit 1: Solutions
Unit 1: Assessment
- true
- false
- false
- true
- true
- true
- false
- false
- false
- false
Media Attributions
- Fig 1 © Libretext is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
a theoretical gas with molecules of negligible size with an average kinetic energy dependent only on temperature
