Mechanical Properties of matter: State, analyse and apply principles of atomic combinations: molecular structure
Unit 1: Molecules and molecular structure
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Use group properties of the periodic table to find the number of valence electrons and valency number of an element.
- Use and draw Lewis diagrams and use the octet rule to explain chemical bonds.
What you should know
Before you start this unit, make sure you can:
- Understand the layout of the periodic table. Refer to level 2 subject outcome 5.3 unit 1 to revise this.
- Understand electron configuration. Refer to level 2 subject outcome 5.3 unit 2 to revise this.
- Understand the structure of groups on the periodic table. Refer to level 2 subject outcome 5.3 unit 3 to revise this.
- Identify and define atoms, ions, and molecules. Refer to level 2 subject outcome 5.4 unit 1 to revise this.
- Understand ionic, covalent, and metallic bonding. Refer to level 2 subject outcome 5.4 unit 3 to revise this.
Introduction[1]
In this unit you will learn about valence electrons, the valence number of an element and how to draw Lewis structures to represent the number of electrons in the outer shells of an atom of the element. Valence electrons are the electrons which orbit the nucleus on the outer level and are involved in forming chemical bonds. The valency number of an element is the number of electrons on the outermost shell. Lewis structures are drawn to show the valence number and follow the octet rule which states that a maximum of eight electrons can be on each level except the first level which can have a maximum of two electrons.
Valence electrons and valency
As you learnt in Level 2, the periodic table is laid out according to increasing atomic number in rows called periods and in horizontal groups because the elements have similar chemical properties. The groups are numbered, with some groups, like group [latex]\scriptsize \displaystyle 1[/latex] the alkali metals and group [latex]\scriptsize \displaystyle 17[/latex] the halogens, given special names. The horizontal rows of the periodic table, from [latex]\scriptsize \displaystyle 1[/latex] to[latex]\scriptsize \displaystyle 7[/latex], are called periods. The period corresponds to the number of electron shells in an atom of that element.
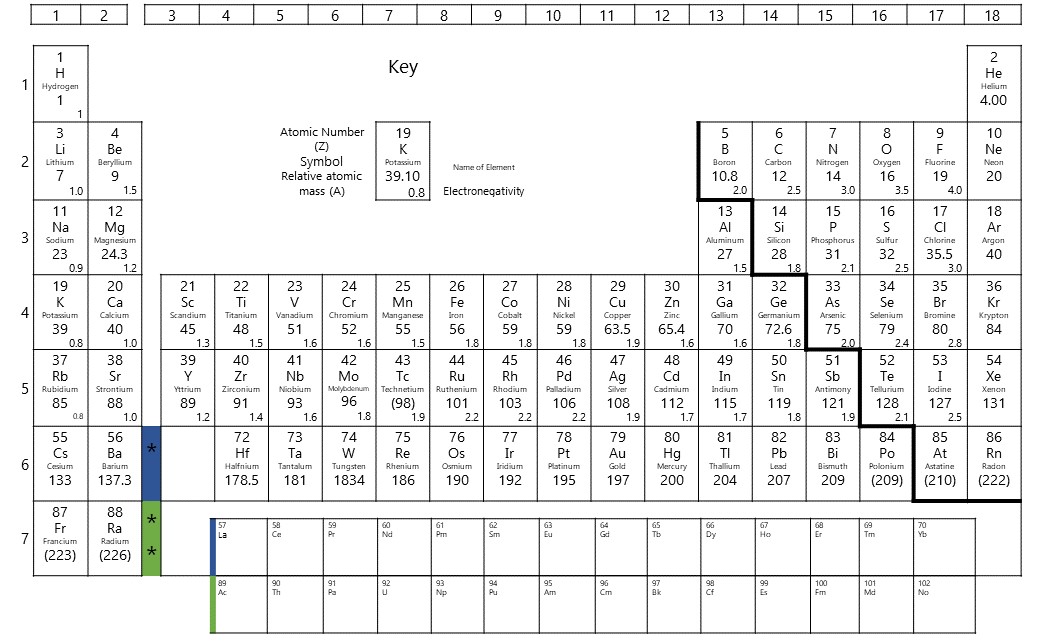
are the electrons an atom has on its outer most energy level, and this is determined using the electron configuration.
Take note!
Calculating valence electrons
Oxygen has an atomic number of [latex]\scriptsize 8[/latex].
This means that a neutral atom of oxygen has [latex]\scriptsize 8[/latex] electrons, so its configuration will be [latex]\scriptsize 2,6[/latex]. (Remember that a maximum of two electrons can fit on the first energy level and [latex]\scriptsize 8[/latex] on the second).
So, oxygen has [latex]\scriptsize 6[/latex] valence electrons.
It is the valence electrons that are involved in bonding. Bonds are formed in order to fill their outer energy levels so that they are more stable. To be stable an atom needs to have [latex]\scriptsize 8[/latex] electrons in its outermost energy level. This is why atoms bond with each other, and their valence electrons influence how many bonds are formed.
Finding valence electrons for all elements
The valence electrons increase in number as you count groups from left to right, along a period. All elements in group [latex]\scriptsize 1[/latex] have atoms with one valence electron; elements in group [latex]\scriptsize \displaystyle 2[/latex] have atoms with two valence electrons; elements in group [latex]\scriptsize \displaystyle 13[/latex] have atoms with three valence electrons; elements in group [latex]\scriptsize \displaystyle 14[/latex] have atoms with four valence electrons; and so forth up to group [latex]\scriptsize \displaystyle 18[/latex]. Elements in group [latex]\scriptsize \displaystyle 18[/latex] have atoms with eight valence electrons, except for helium, which has only two. For groups [latex]\scriptsize \displaystyle 13[/latex] to [latex]\scriptsize \displaystyle 10[/latex], the number of valence electrons is the group number minus [latex]\scriptsize \displaystyle 10[/latex].
The transition metals, groups , do not follow these rules. Transition metals are more complicated because they can have incomplete shells; they do not follow the rule of [latex]\scriptsize \displaystyle 2,8,8,2[/latex] in their arrangement of electrons. Their valency can vary depending on the other atoms they bond with. The valency is indicated using a stock number (roman numeral) in brackets in the name of the compound, for example copper (II) oxide. Copper has a valency of [latex]\scriptsize \displaystyle 2[/latex] in this compound.
Valency
The of an atom is the number of bonds it can form. Valency is important to know because it allows you to work out the ratio in which atoms will bond with other atoms. Remember, atoms bond to complete their outer energy levels, either by giving away electrons in their outer most energy level (becoming cations), gaining electrons to fill up their outermost energy level (becoming anions), or by sharing electrons (forming molecules).
There is a relationship between the valency of an element and the number of valence electrons. For groups [latex]\scriptsize \displaystyle 1,\text{ }2,\text{ }13[/latex] and [latex]\scriptsize \displaystyle 14[/latex], the valency is equal to the number of valence electrons. These elements tend to lose electrons to revert to an inner energy level that is full. Their valency is given a positive sign.
For groups [latex]\scriptsize \displaystyle 15[/latex] to [latex]\scriptsize \displaystyle 18[/latex], the valency is equal to [latex]\scriptsize 8[/latex] minus the number of valence electrons. These elements tend to gain electrons in order to fill up their outer energy level. Their valency is given a negative sign. Elements in group [latex]\scriptsize \displaystyle 18[/latex] have atoms with full outer energy levels; therefore their valency is [latex]\scriptsize 0[/latex]. They do not form bonds with other atoms. For the transition metals, the valency can vary. In these cases, we indicate the valency by a roman numeral after the name of the metal in the compound, for example iron (III) chloride.
Example 1.1
Calculate the valency of the following non-metals:
- carbon in group [latex]\scriptsize \displaystyle 14[/latex]
- nitrogen in group [latex]\scriptsize \displaystyle 15[/latex].
Solution
- The valency of carbon is group [latex]\scriptsize \displaystyle 14\text{ -}10\text{ =}~4[/latex], so carbon has [latex]\scriptsize 4[/latex] valence electrons.
.
The atomic number is [latex]\scriptsize 6[/latex], which means [latex]\scriptsize 6[/latex] protons and [latex]\scriptsize 6[/latex] electrons.
The electron configuration is [latex]\scriptsize 2,4[/latex].
.
To have a full outer energy level carbon needs to gain [latex]\scriptsize \displaystyle 4[/latex]electrons because [latex]\scriptsize 8-4=4[/latex].
.
Therefore, carbon has a valency of [latex]\scriptsize \displaystyle 4[/latex]. This means it can form [latex]\scriptsize 4[/latex] bonds. - The valency of nitrogen is group [latex]\scriptsize \displaystyle 15-10=5[/latex], so nitrogen has [latex]\scriptsize 5[/latex] valance electrons, [latex]\scriptsize 8-5=3[/latex], therefore valency of [latex]\scriptsize 3[/latex].
.
The atomic number is [latex]\scriptsize \displaystyle 7[/latex] which means it has [latex]\scriptsize \displaystyle 7[/latex] electrons and [latex]\scriptsize \displaystyle 7[/latex] protons.
.
The electron configuration is [latex]\scriptsize 2,5[/latex] (so there are [latex]\scriptsize 5[/latex] electrons in the outer energy level).
To have a full outer energy level of [latex]\scriptsize 8[/latex] electrons, a nitrogen atom needs to gain [latex]\scriptsize 3[/latex] more electrons: [latex]\scriptsize 8-5=3[/latex].
.
Therefore, nitrogen has a valency of [latex]\scriptsize 3[/latex] and it can form [latex]\scriptsize 3[/latex] bonds.
Exercise 1.1
Copy the following table into your notebook and then complete it:
| Element | Group Number | Number of Valence | Electrons Number of electrons needed (gained or lost) for a full outer energy level | Valency (+/-) |
| Cl | ||||
| Li | ||||
| P | ||||
| S | ||||
| Ne | ||||
| Mg | ||||
| Al | ||||
| C | ||||
| N | ||||
| O |
The full solutions can be found at the end of the unit.
Bonding and Lewis structures
In level 2, subject outcome 5.3, unit 3, you learnt about how elements bond. Atoms bond in order to become stable, like the noble gases. The noble gases have a full valence electron energy level. For example, neon has the following electronic configuration: [latex]\scriptsize 2,8[/latex]. The second energy level is the outermost (valence) shell and is full.
When different atoms bond they form new compounds. Compounds can have a mixture of single, double, and triple bonds and an atom can have several bonds. In other words, an atom does not need to share all its valence electrons with one other atom but can share its valence electrons with several different atoms. Atoms that have at least one unpaired electron can form a bond with any other atom that also has an unpaired electron. A pair of electrons, one from each atom that form a bond are called a bonding pair. This is not restricted to just two atoms.
Not all the electrons on the outermost energy level are involved in forming bonds. The valence electrons not involved in bonding are called .
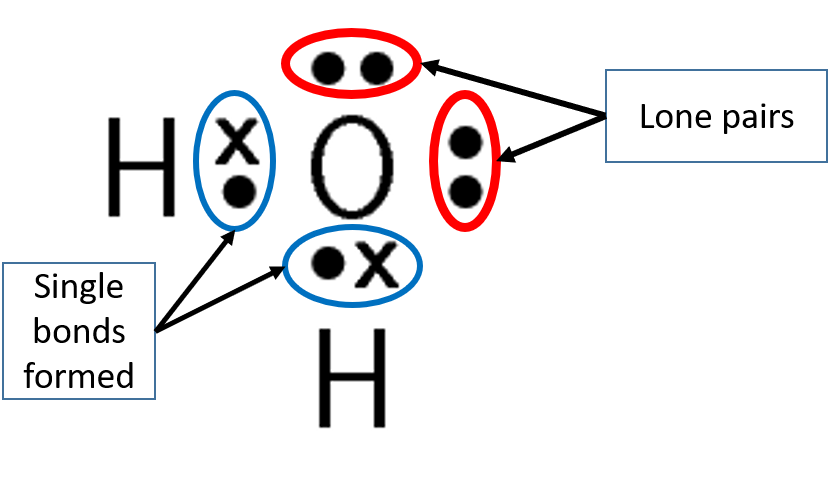
Take note!
- Atoms form bonds to try to achieve the same electron configuration as the noble gases.
- Atoms with a full valence electron energy level are less reactive as they do not form bonds to fill their outer energy levels.
- The octet rule states that a maximum of [latex]\scriptsize 2[/latex] or [latex]\scriptsize 8[/latex] electrons can be on the outer most energy level depending on the atom.
- Only valence electrons are involved in bonding.
- During bonding, atoms will gain, lose, or share electrons to have a full outer energy level and therefore become a stable compound.
- Valency is the number of electrons an atom needs to lose or gain to have a full outer shell.
Covalent bonding
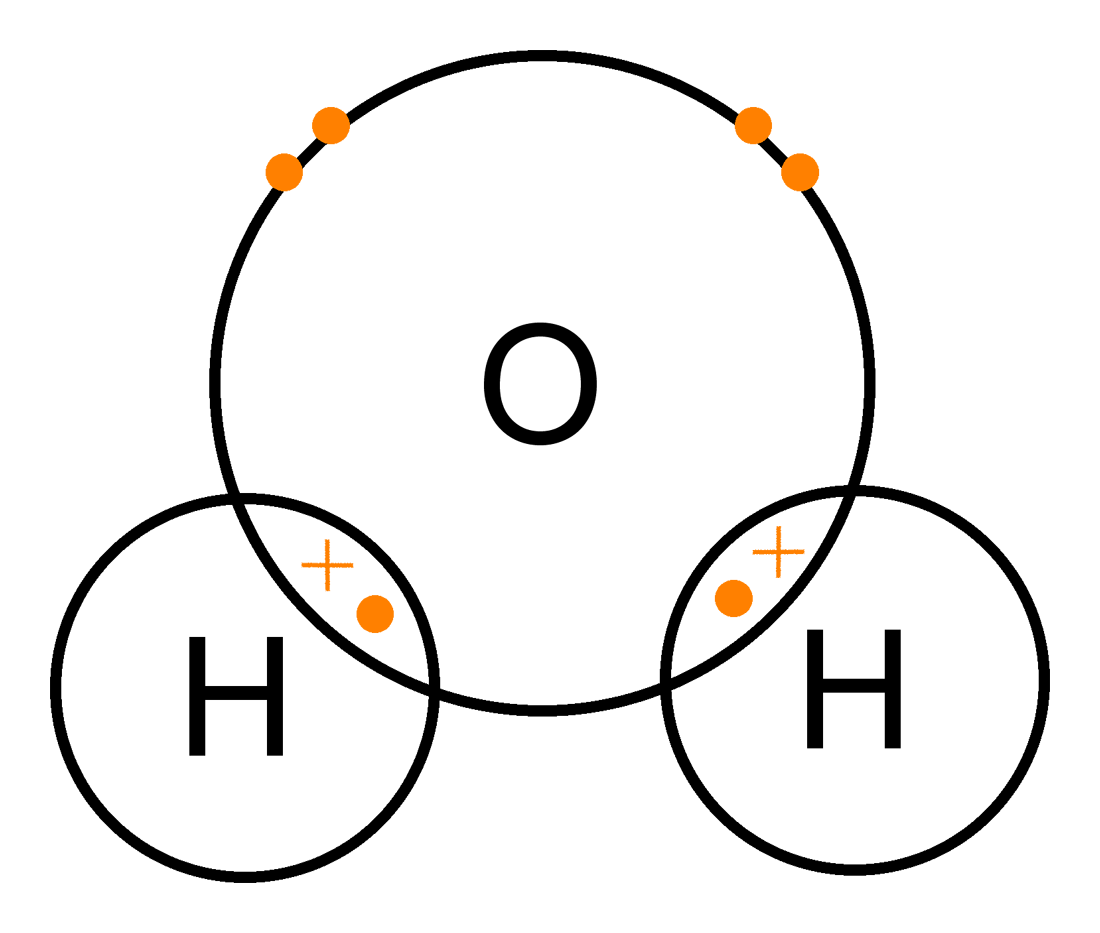
Non-metals will bond with each other by sharing electrons on their outer most energy level. This is called . The outermost orbitals of the atoms overlap so that unpaired electrons in each of the bonding atoms can be shared. By overlapping orbitals, the outer energy shells of all the bonding atoms are filled. The shared electrons move in the orbitals around both atoms. As they move, there is an attraction between these negatively charged electrons and the positively charged nuclei. This attractive force holds the atoms together in a covalent bond.
Ionic bonding
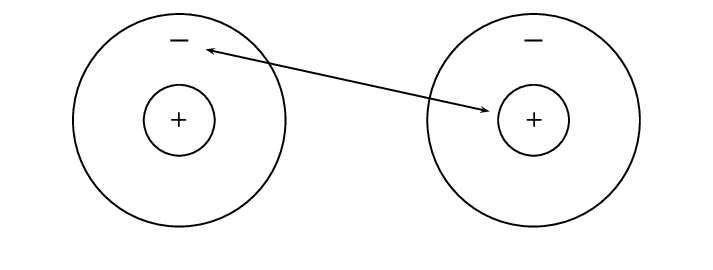
Metals and non-metals will bond by gaining or losing electrons and this is called ionic bonding. Ionic bonding takes place when the difference in electronegativity between the two atoms is more than [latex]\scriptsize \displaystyle 1,7[/latex]. This usually happens when a metal atom bonds with a non-metal atom. When the difference in electronegativity is large, one atom will attract the shared electron pair much more strongly than the other, causing electrons to be transferred to the atom with higher electronegativity. When ionic bonds form, a metal donates one or more electrons, due to having a low electronegativity, to form a positive ion or cation. The non-metal atom has a high electronegativity, and therefore readily gains electrons to form a negative ion or anion. The two ions are then attracted to each other by electrostatic forces.
Lewis structures
use dots and crosses to represent the valence electrons on different atoms. The chemical symbol of the element is used to represent the nucleus and the inner electrons of the atom. To determine which are the valence electrons we look at the outermost energy level in the atom’s electron structure.
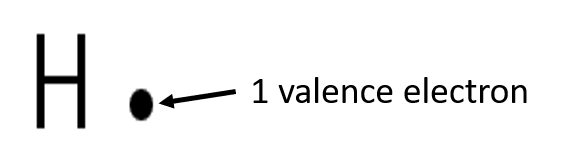
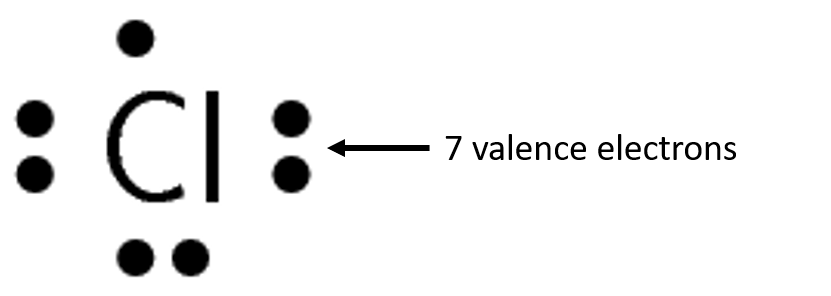
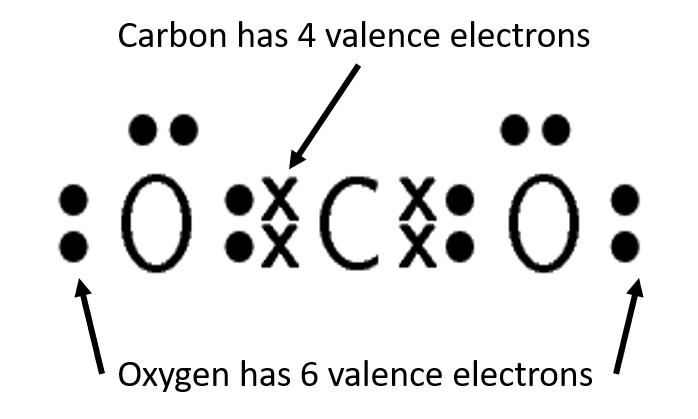
Exercise 1.2
Represent the following atoms as Lewis structures:
- Lithium
- Nitrogen
- Helium
The full solutions can be found at the end of the unit.
Lewis structures and covalent bonds
A single covalent bond is formed when two electrons are shared between the same two atoms, one electron from each atom.
A double covalent bond is formed when four electrons are shared between the same two atoms, two electrons from each atom. An example of a double covalent bond can be seen in figure 7.
A triple covalent bond is formed when six electrons are shared between the same two atoms, three electrons from each atom.
Let’s look at an example of single covalent bonding.
Example 1.2
How do hydrogen and chlorine atoms bond covalently in a molecule of hydrogen chloride?
Solution
Step 1: Determine the electron configuration of each of the bonding atoms
A chlorine atom has [latex]\scriptsize 17[/latex] electrons and an electron configuration of [latex]\scriptsize \displaystyle 2,8,7[/latex] therefore, chlorine has [latex]\scriptsize \displaystyle 7[/latex] valence electrons and a valency of [latex]\scriptsize 1[/latex]. A hydrogen atom has only one electron and an electron configuration of [latex]\scriptsize 1[/latex]. Therefore, hydrogen has [latex]\scriptsize 1[/latex] valence electron and a valency of [latex]\scriptsize 1[/latex].
Step 2: Determine how many of the electrons are paired or unpaired
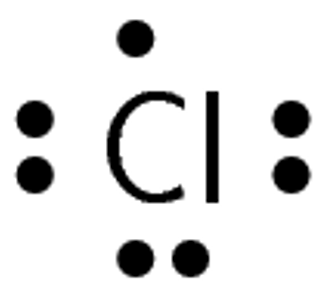
Chlorine has seven valence electrons. One of these electrons is unpaired.
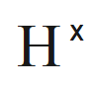
Hydrogen has one valence electron, and it is unpaired.
Step 3: Work out how the electrons are shared
The hydrogen atom needs one more electron to complete its outermost energy level. The chlorine atom also needs one more electron to complete its outermost energy level. Therefore, one pair of electrons must be shared between the two atoms. A single covalent bond will be formed.
Let’s look at an example of covalent bonding involving multiple bonds.
Example 1.3
How do nitrogen and hydrogen atoms bond to form a molecule of ammonia (NH3)?
Solution
Step 1: Give the electron configuration
A nitrogen atom has seven electrons, and an electron configuration of [latex]\scriptsize \displaystyle 2,5[/latex]. A hydrogen atom has only one electron, and an electron configuration of [latex]\scriptsize \displaystyle 1[/latex].
Step 2: Give the number of valence electrons
Nitrogen has five valence electrons. Three of these electrons are unpaired (they are lone electrons). Hydrogen has one valence electron, and it is unpaired.
Step 3: Work out how the electrons are shared
Each hydrogen atom needs one more electron to complete its valence energy shell. The nitrogen atom needs three more electrons to complete its valence energy shell. Therefore, three pairs of electrons must be shared between the four atoms involved. Three single covalent bonds will be formed.
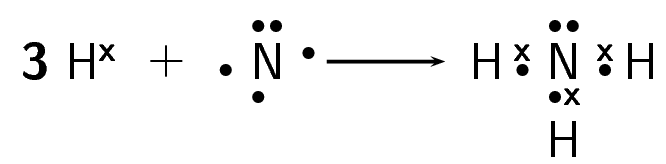
Now let’s look at an example of covalent bonding involving a double bond.
Example 1.4
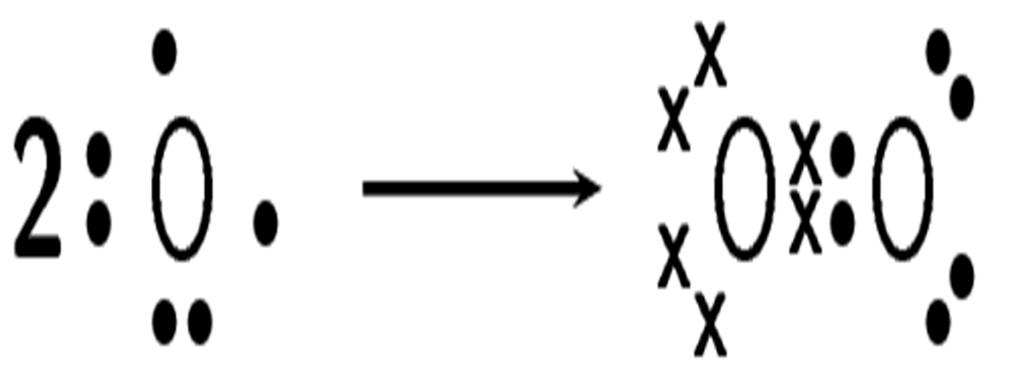
How do oxygen atoms bond covalently to form an oxygen molecule?
Solution
Step 1: Determine the electron configuration of the bonding atoms
Each oxygen atom has eight electrons, and their electron configuration is [latex]\scriptsize \displaystyle 2,6[/latex].
Step 2: Determine the number of valence electrons for each atom and how many of these electrons are paired and unpaired
Each oxygen atom has six valence electrons. Each atom has two unpaired electrons.
Step 3: Work out how the electrons are shared
Each oxygen atom needs two more electrons to complete its valence energy shell. Therefore, two pairs of electrons must be shared between the two oxygen atoms so that both outermost energy levels are full. A double bond is formed.
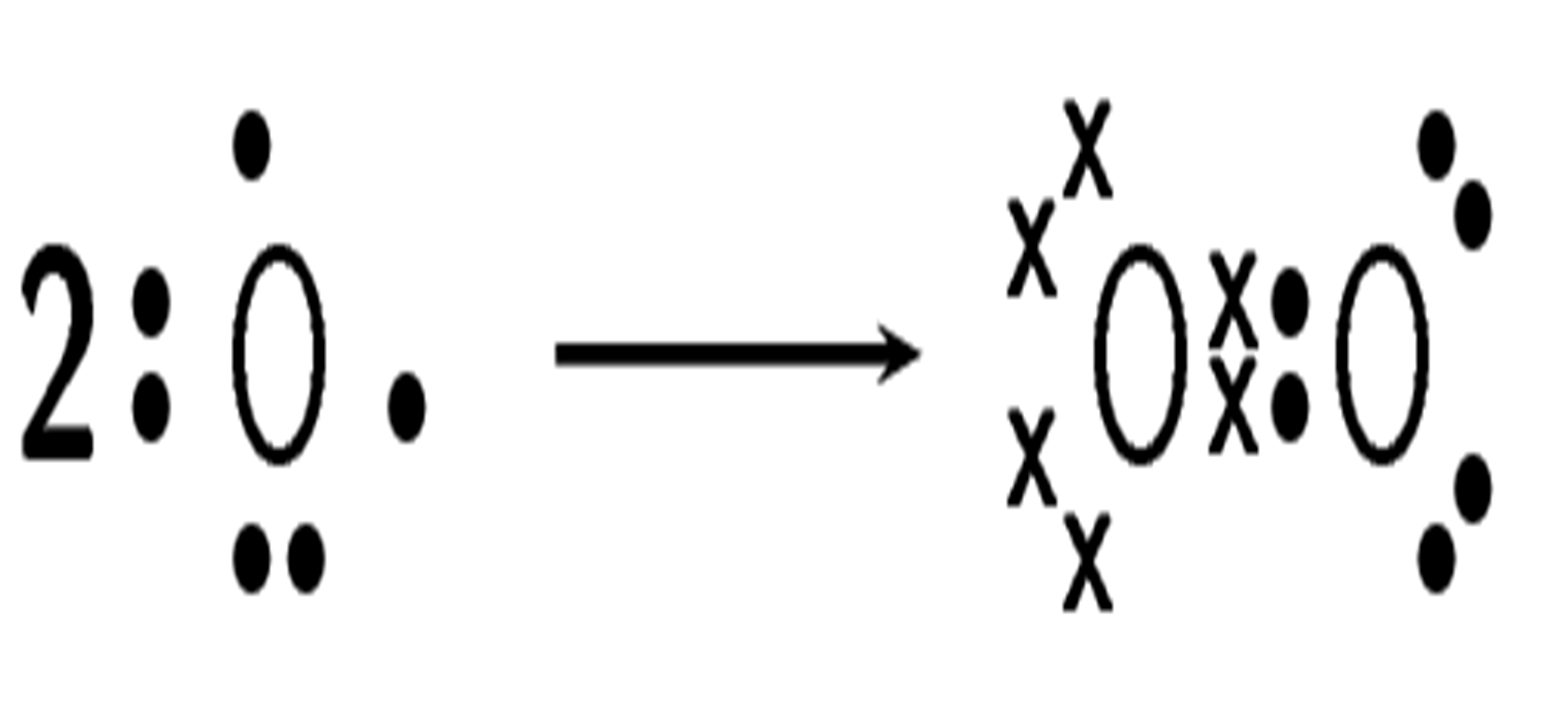
Here is an example looking at triple bonds in hydrogen cyanide (HCN).
Example 1.5
Hydrogen cyanide (HCN) as triple bonds.
- For each atom, determine the number of valence electrons that the atom has from its electron configuration.
- Arrange the electrons in the HCN molecule so that the outermost energy level in each atom is full.
Solutions
- The electron configuration of hydrogen is [latex]\scriptsize 1[/latex], the electron configuration of nitrogen is [latex]\scriptsize 2,5[/latex] and for carbon is [latex]\scriptsize 2,4[/latex]. Hydrogen has [latex]\scriptsize 1[/latex] valence electron; carbon has [latex]\scriptsize 4[/latex] valence electrons and nitrogen has [latex]\scriptsize 5[/latex] valence electrons.
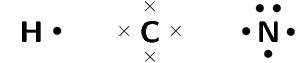
- The HCN molecule is represented below. Notice the three electron pairs (highlighted in red) between the nitrogen and carbon atom. Because these three covalent bonds are between the same two atoms, this is a triple bond.

.
Lines can be used to represent the bonds formed between atoms as shown in the diagram below. A line represents a shared pair of electrons and these diagrams are called Couper structures.
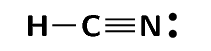
Exercise 1.3
- Explain the bonds formed in water by answering the following questions:
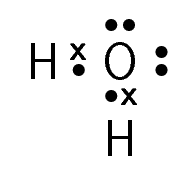
- What is the electron configuration of oxygen?
- What is the electron configuration of hydrogen?
- How many valence electrons do oxygen are hydrogen have?
- State the number and kinds of bonds formed.
- Explain the difference between the number of valence electrons and the valency of an element.
The full solutions can be found at the end of the unit.
Lewis structures and ionic bonds
When electrons are transferred from one atom to another it is called . Metals tend to lose electrons and become anions which are positively charged, and non-metals gain electrons to become anions which are negatively charged.
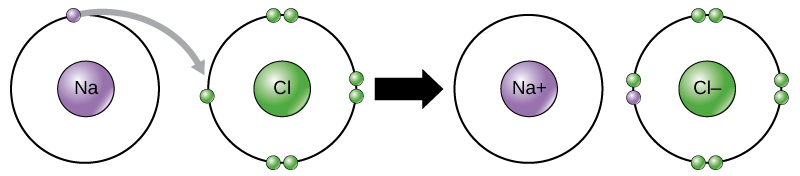
Example 1.6
- How does ionic bonding occur in sodium chloride?
- Give the balanced equation for this reaction.
Solutions
- The difference in electronegativity between Na [latex]\scriptsize (0.93)[/latex] and Cl [latex]\scriptsize (3.16)[/latex] is [latex]\scriptsize 2.1[/latex].
.
Sodium has only one valence electron, while chlorine has seven.
.
Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. This electron from sodium is transferred to chlorine. Sodium loses an electron and forms an [latex]\scriptsize [\text{N}{{\text{a}}^{+}}][/latex] ion.
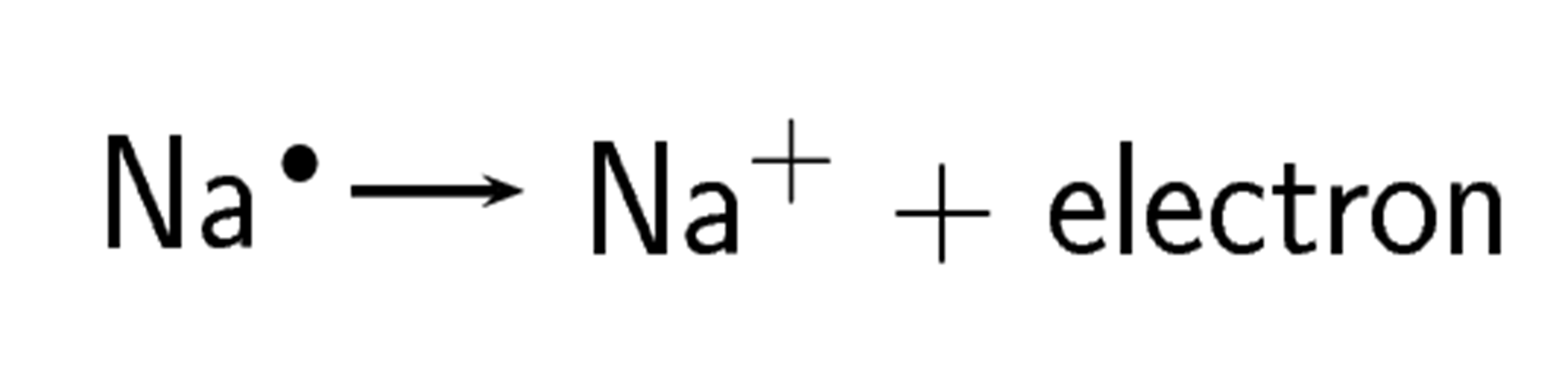
.
Chlorine gains an electron and forms a [latex]\scriptsize [\text{C}{{\text{l}}^{-}}][/latex] ion:

.
The electron is therefore transferred from sodium to chlorine:
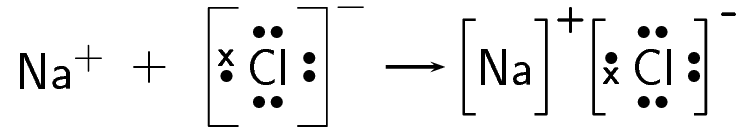
- The balanced equation for this reaction is: [latex]\scriptsize 2\text{Na + C}{{\text{l}}_{{_{{{{2}_{{}}}}}}}}\to 2\text{NaCl}[/latex].
Example 1.7
- How does ionic bonding occur in magnesium oxide?
- What holds the compound together?
Solutions
- Magnesium has two valence electrons and an electronegativity of [latex]\scriptsize 1.31[/latex], while oxygen has six valence electrons and an electronegativity of [latex]\scriptsize 3.44[/latex].
.
Since oxygen has a higher electronegativity, it attracts the two valence electrons from the magnesium atom and these electrons are transferred from the magnesium atom to the oxygen atom.
.
Magnesium loses two electrons to form Mg2+, and oxygen gains two electrons to form O2-.
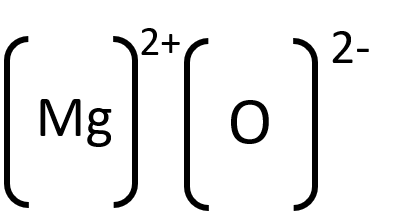
- The attractive force between the oppositely charged ions is what holds the compound together MgO.
The crystal lattice structure of ionic compounds
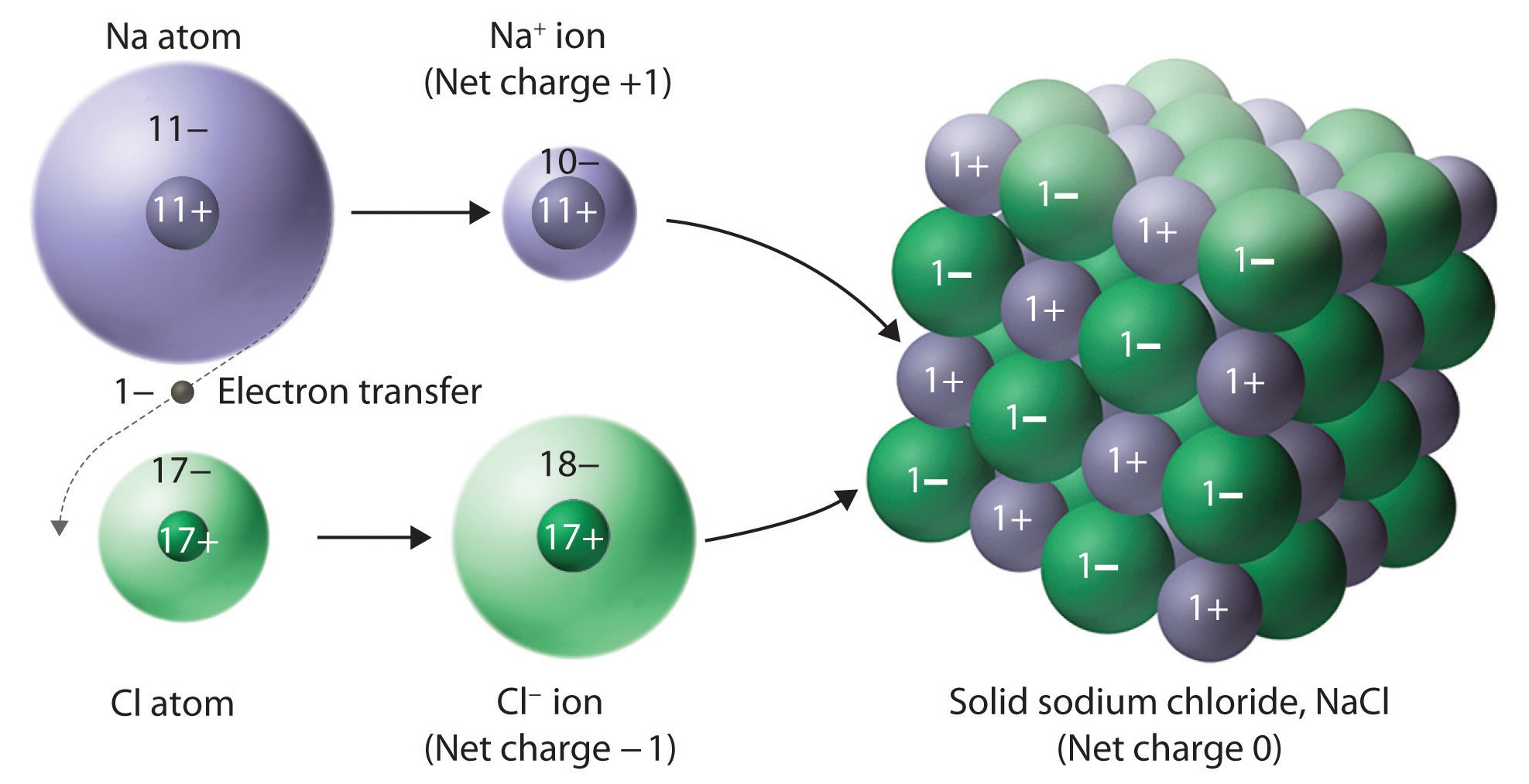
Ionic substances are a combination of many ions bonded together into a giant molecule. The arrangement of ions in a regular, geometric structure is called a crystal lattice.
Sodium chloride does not contain one chlorine ion and one sodium ion, but rather millions of these two ions arranged in a crystal lattice where the ratio of sodium to chlorine ions is [latex]\scriptsize \displaystyle 1:1[/latex]. The structure of the crystal lattice is shown in figure 9.
Exercise 1.4
Draw Lewis diagrams to represent the following ionic compounds:
- sodium iodide (NaI)
- calcium bromide (CaBr2)
- potassium chloride (KCl)
The full solutions can be found at the end of the unit.
Summary
In this unit you have learnt the following:
- Atoms will form bonds by gaining, sharing, or losing electrons to gain a stable electron configuration.
- The valence electrons are the electrons in the outermost energy level and are involved in bonding. The number of valence electrons can be calculated by using the group number of the element in the periodic table.
- The valency of an element is the number of bonds it can form.
- If an atom has more than one unpaired electron it can form multiple bonds to another atom. In this way double and triple bonds are formed.
- A lone pair is an electron pair in the outermost level of an atom that is not shared or bonded to another atom.
- Covalent bonds are formed between non-metals and involve sharing electrons.
- Ionic bonds are formed between metals and non-metals and involve the loss or gain of electrons.
- Metals will lose electrons to become positively charged cations.
- Non-metals will gain electrons to become negatively charged anions.
- Lewis structures are diagrams drawn to show the bonds formed and the lone pairs, where dots and crosses are used to represent the electrons involved from different elements.
Unit 1: Assessment
Suggested time to complete: 30 minutes
- Explain the difference between a covalent bond and an ionic bond.
- Draw Lewis diagrams to represent the following:
- argon (Ar)
- chlorine (Cl2)
- methane (CH4)
- oxygen difluoride (OF2)
- Magnesium and chlorine react to form magnesium chloride (MgCl2). The electronegativity of magnesium is: [latex]\scriptsize 1.31[/latex]. The electronegativity of chlorine is [latex]\scriptsize 3.16[/latex].
- Calculate the difference in electronegativity.
- Use the difference in electronegativity to explain what type of bonding will form between these two elements and state which element will lose electrons and which will gain electrons.
- Draw a Lewis structure to show the bonding in MgCl2.
- Hydrogen will readily bond with nitrogen to form the gas ammonia (NH3).
- For this reaction give the number of:
- valence electrons for each atom involved in the reaction.
- bonds each atom can form.
- Draw a Lewis diagram of the product formed.
- For this reaction give the number of:
- A chemical compound has the following Lewis diagram:
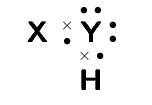
- How many valence electrons does element Y have?
- How many valence electrons does element X have?
- How many covalent bonds are in the molecule?
- Suggest a name for the elements X and Y.
- Copy the following table into your notebook and complete it:
Compound CO2 CF4 HI C2H2 Lewis diagram Total number of bonding pairs Total number of lone pairs Single, double, or triple bonds
The full solutions can be found at the end of the unit.
Unit 1: Solutions
Exercise 1.1
The number of valence electrons are the electrons on the outermost energy level. The valency is the number of bonds an element can form, and it is the same number as the number of electrons an atom needs to lose, gain or share to have a full outer energy level.
| Element | Group Number | Number of Valence | Electrons Number of electrons needed (gained or lost) for a full outer energy level | Valency (+/-) |
| Cl | [latex]\scriptsize 17[/latex] | [latex]\scriptsize 7[/latex] | [latex]\scriptsize 1[/latex] | [latex]\scriptsize +1[/latex] |
| Li | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 1[/latex] | [latex]\scriptsize -1[/latex] |
| P | [latex]\scriptsize 15[/latex] | [latex]\scriptsize 5[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize +3[/latex] |
| S | [latex]\scriptsize 16[/latex] | [latex]\scriptsize 6[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize +2[/latex] |
| Ne | [latex]\scriptsize 18[/latex] | [latex]\scriptsize 8[/latex] | [latex]\scriptsize 0[/latex] | [latex]\scriptsize 0[/latex] |
| Mg | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize -2[/latex] |
| Al | [latex]\scriptsize 13[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize -3[/latex] |
| C | [latex]\scriptsize 14[/latex] | [latex]\scriptsize 4[/latex] | [latex]\scriptsize 4[/latex] | [latex]\scriptsize +4[/latex] |
| N | [latex]\scriptsize 15[/latex] | [latex]\scriptsize 5[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize +3[/latex] |
| O | [latex]\scriptsize 16[/latex] | [latex]\scriptsize 6[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize +2[/latex] |
Exercise 1.2
- Lithium
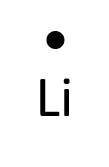
- Nitrogen
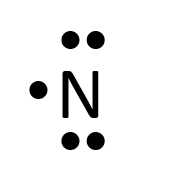
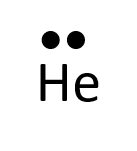
- Helium
Exercise 1.3
- .
- [latex]\scriptsize 2,6[/latex]
- [latex]\scriptsize 1[/latex]
- Valence electrons of oxygen: [latex]\scriptsize 6[/latex]
Valence electrons of hydrogen: [latex]\scriptsize 1[/latex] - Two covalent bonds are formed.
- Valence electrons are the electrons on the atom’s outer energy level and the valency is the number of bonds the atom can form.
Exercise 1.4
- sodium iodide (NaI)
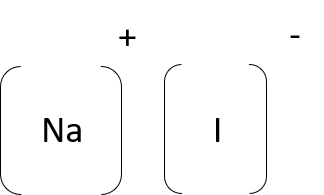
- calcium bromide (CaBr2)
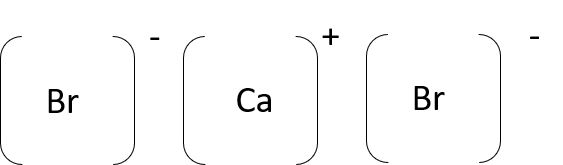
- potassium chloride (KCl)
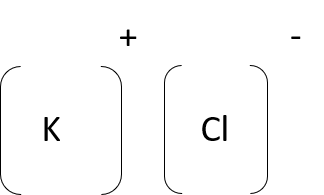
Unit 1: Assessment
- A covalent bond occurs when valence electrons are shared by the atoms which are bonding together. Covalent bonds occur between non-metals.
.
Ionic bonding happens when a metal donates electrons to a non-metal. This means that the metal will become positively charged and the non-metal will become negatively charged. - .
- Argon
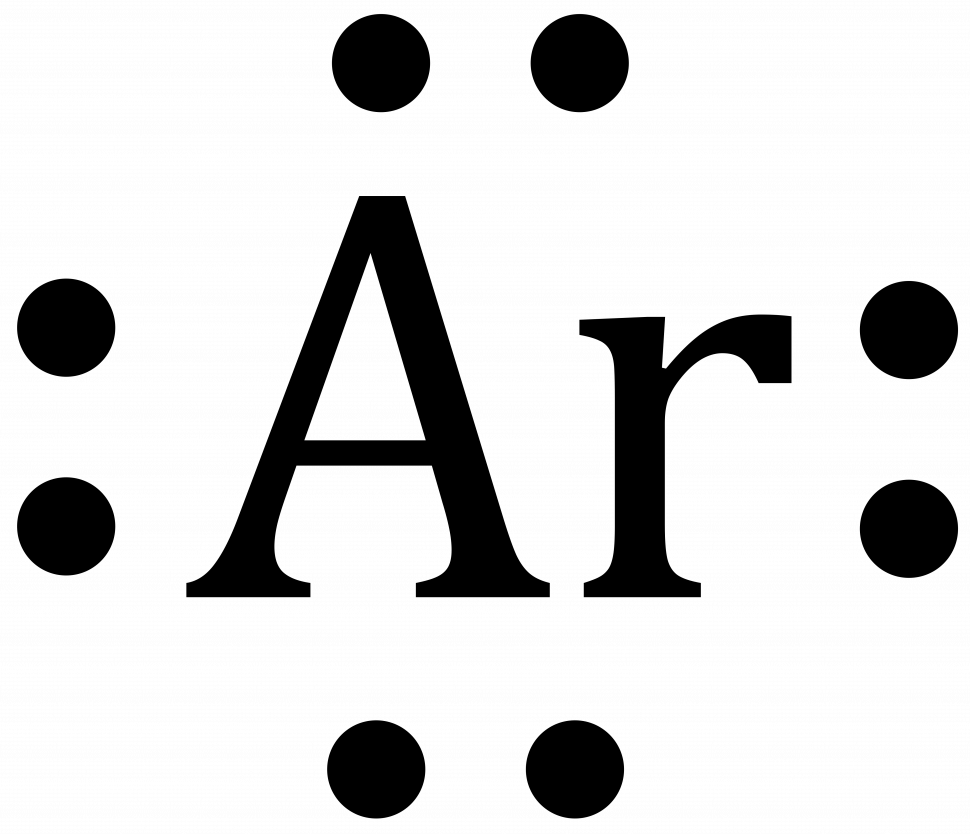
- Chlorine
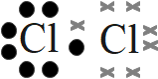
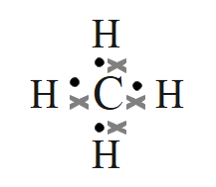
- Methane
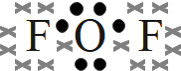
- oxygen difluoride
- Argon
- Magnesium and chlorine react to form magnesium chloride (MgCl2).
- [latex]\scriptsize 3.16-1.31=1.85[/latex]
- Because the electronegativity is [latex]\scriptsize 1.85[/latex] an ionic bond will form. This is because the electronegativity is higher than [latex]\scriptsize 1.7[/latex] and a metal is involved in the formation of the compound.
Magnesium will lose [latex]\scriptsize 2[/latex] electrons and each chlorine atom will gain [latex]\scriptsize 1[/latex] electron. - .
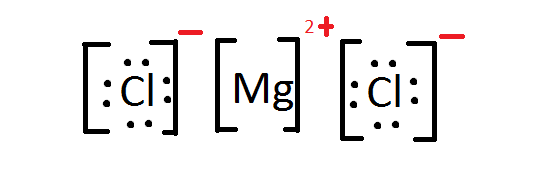
- .
- .
- Each hydrogen atom has [latex]\scriptsize 1[/latex] valence electron, and nitrogen has [latex]\scriptsize 5[/latex].
- Each hydrogen atom can form [latex]\scriptsize 1[/latex] bond and the nitrogen can form [latex]\scriptsize 3[/latex].
- .
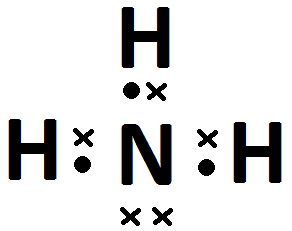
- .
- .
- [latex]\scriptsize 6[/latex]
- [latex]\scriptsize 1[/latex]
- [latex]\scriptsize 2[/latex]
- X – hydrogen, Y – oxygen or sulphur
- .
Compound CO2 CF4 HI C2H2 Lewis diagram 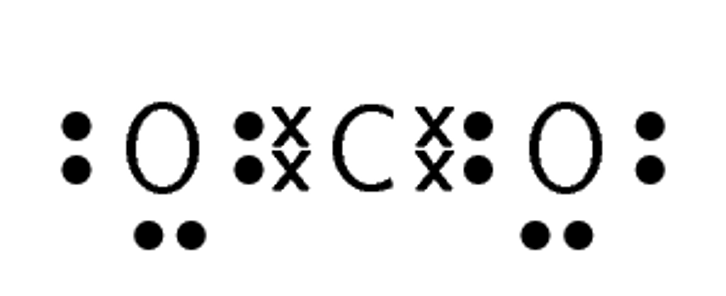
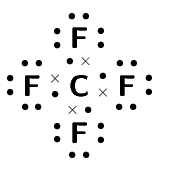
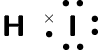
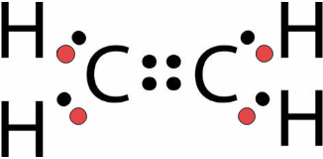
Total number of bonding pairs [latex]\scriptsize 4[/latex] [latex]\scriptsize 4[/latex] [latex]\scriptsize 1[/latex] [latex]\scriptsize 5[/latex] Total number of lone pairs [latex]\scriptsize 4[/latex] [latex]\scriptsize 12[/latex] [latex]\scriptsize 3[/latex] [latex]\scriptsize 0[/latex] Single, double, or triple bonds Two double bonds Four single bonds One single bond One triple bond and two single bonds
Media Attributions
- Fig 1 © DHET is licensed under a CC BY (Attribution) license
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
- Fig 3 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 4 © Benjah-bmm27 is licensed under a CC0 (Creative Commons Zero) license
- Fig 5 © DHET is licensed under a CC BY (Attribution) license
- Fig 6 © DHET is licensed under a CC BY (Attribution) license
- Fig 7 © DHET is licensed under a CC BY (Attribution) license
- Fig 8 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 9 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 12 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 11 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 13 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 14 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 15 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 16 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 17 © Libretext is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 18 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 19 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 21 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 23 © DHET is licensed under a CC BY (Attribution) license
- Fig 24 © Libretext is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- fig 25 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- fig 26 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 27 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- fig 28 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 30 © DHET is licensed under a CC BY (Attribution) license
- Fig 31 © DHET is licensed under a CC BY (Attribution) license
- Fig 32 © DHET is licensed under a CC BY (Attribution) license
- Fig 33 © Andrewbdfe is licensed under a CC0 (Creative Commons Zero) license
- Fig 34 © DHET is licensed under a CC BY (Attribution) license
- Fig 35 © DHET is licensed under a Public Domain license
- Fig 36 © DHET is licensed under a CC BY (Attribution) license
- Fig 37 © Libretext is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 38 © DHET is licensed under a CC BY (Attribution) license
- Fig 39 © DHET is licensed under a CC BY (Attribution) license
- Fig 40 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 41 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 42 © DHET is licensed under a CC BY (Attribution) license
- Parts of the text in this unit were sourced from Siyavula Physical Science Gr 11 Learner’s Book, Chapter 3, released under a CC-BY licence. ↵
the number of electrons on the outermost energy level
the number of bonds an atom can form
electron pairs on the outermost energy level which are not involved in bonding
type of chemical bonding where electrons on the outermost energy level are shared
using dots and crosses to represent the valence electrons in an atom
type of chemical bonding where one or more electrons are transferred from one atom to another
