Mechanical Properties of matter: State, analyse and apply principles of atomic combinations: molecular structure
Unit 5: Polar and non-polar substances
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Identify polar and non-polar substances.
What you should know
Before you start this unit, make sure you can:
- Understand molecules and molecular structure. Refer to level 3 subject outcome 5.2 unit 1 to revise this.
- Understand electronegativity and polarity. Refer to level 3 subject outcome 5.2 unit 2 to revise this.
- Understand shapes of molecules. Refer to level 3 subject outcome 5.2 unit 4 to revise this.
Introduction
In this unit you will learn about polar and non-polar substances. A polar substance is one which contains molecules that have oppositely charged sides. This arises when the covalent bonds are polar because electrons are not equally shared, and the shape of the molecule is asymmetrical. Non-polar substances have molecules that do not have oppositely charged sides because the covalent bonds are pure (electrons are shared equally) or they have polar bonds, but the molecular shape is symmetrical. Ionically bonded substances are also considered to be polar and there are separate ions with opposite charges.
Polar and non-polar (pure) covalent bonds
It is important to be able to determine if a molecule is polar or non-polar since the of molecules affects properties such as solubility, melting points and boiling points.
Polarity occurs when an atom or a molecule has both positive and negative charges, and it is the separation of the electric charge which leads a molecule to have a positive and negative end. The bond or the molecular polarities depend upon the electronegativities of the atoms or the molecules as well as the molecular shape.
As we learnt in unit 2 can be used to explain the difference between two types of covalent bonds. If the electronegativity is less than [latex]\scriptsize 0.4[/latex] then the bond formed is called a pure covalent bond, if the electronegativity is below [latex]\scriptsize 1.7[/latex] the bond formed is polar and if the electronegativity difference is above [latex]\scriptsize 1.7[/latex] the bond formed is ionic. Remember that bonds with an electronegative difference above [latex]\scriptsize 1.6[/latex] and a metal element is involved, then the bond formed will be ionic.
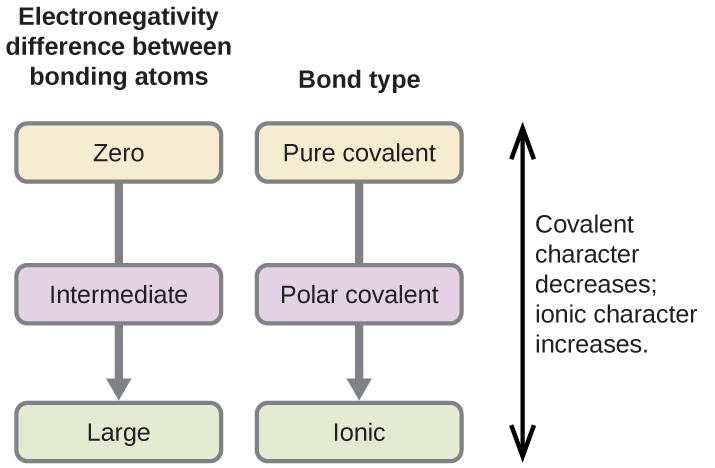
| Electronegativity difference | Type of bond |
| [latex]\scriptsize 0[/latex] | Pure covalent |
| [latex]\scriptsize 0-1[/latex] | Weak polar covalent |
| [latex]\scriptsize 1.1-1.9[/latex] | Strong polar covalent |
| [latex]\scriptsize \ge 2.0[/latex] | Ionic |
form between two identical non-metal atoms, for example H2, Cl2 and O2. Because the two atoms have the same electronegativity, the electron pair in the covalent bond is shared equally between them.
If two different non-metal atoms bond, then the shared electron pair will be pulled more strongly by the atom with the higher electronegativity. As a result, a is formed where one atom will have a slightly negative charge and the other a slightly positive charge.
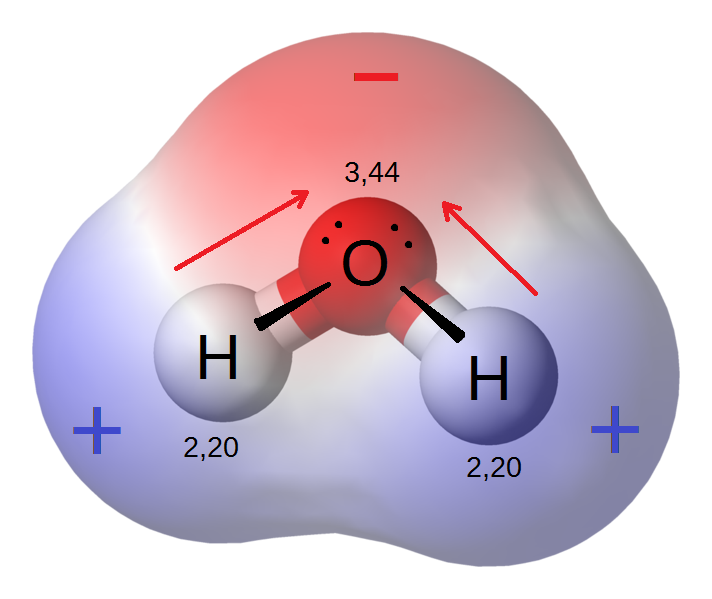
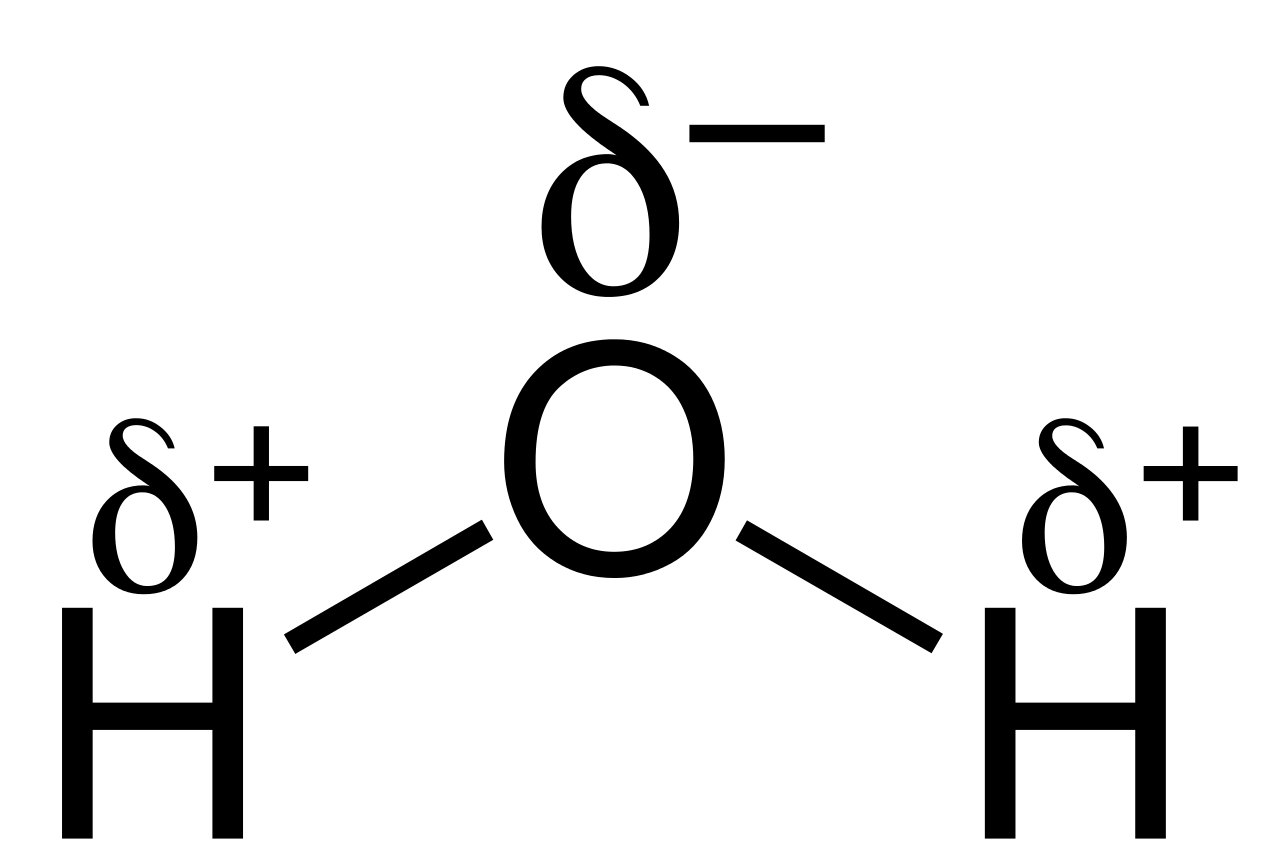
In a water molecule (see figure 2), the electrons from the two hydrogen atoms will spend more time orbiting the oxygen atom because it has a higher electronegativity.
This slightly positive or slightly negative charge is known as a partial charge. These partial charges are represented using the symbols δ+ (slightly positive) and δ− (slightly negative).
The δ symbol is pronounced ‘delta’
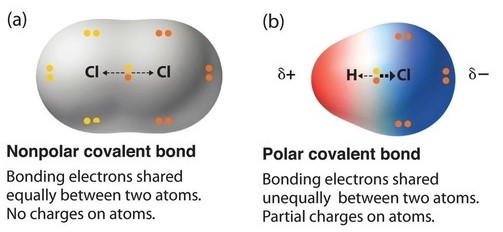
Polar and non-polar molecules
A is one that does have sides with opposite charge. Either the bonds are non-polar or it is a symmetrical molecule with polar bonds. Examples include carbon dioxide, oxygen and methane. Simply put, a non-polar bond is formed when the electronegativity difference is zero.
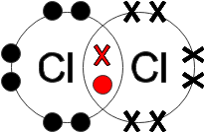
In figure 4, you can see the electrons being shared between two chlorine atoms. Because the electronegativity of the atoms is the same, the electrons will spend an equal amount of time close to both nuclei and they will both have a neutral charge. A pure covalent bond is formed by the equally strong electrostatic attraction between the shared pair of electrons and the nuclei of the bonded atoms. The molecule does not have oppositely charged ends and is therefore non-polar.
Methane is another example of a non-polar molecule. Methane’s formula is CH4. It forms a symmetrical tetrahedral shape when bonded, and the C-H bonds have an electronegativity difference of [latex]\scriptsize 0.4[/latex] so the bonds are polar. The H atoms will have a slightly positive charge. However, because of the symmetrical shape, the molecule does not have oppositely charged sides, therefore it is non-polar.
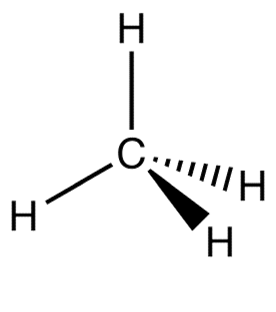
A is usually formed when the one end of the molecule is said to possess a positive charge and the opposite end of the molecule has a negative charge, creating electrical polarity. When a molecule has polar bonds, and the shape of the molecule is asymmetrical (linear with different atoms, pyramidal and angular), then the entire molecule will be a polar molecule.
Water is the most common polar molecule. As described in figure 2, water is polar because the central atom, oxygen, has a higher electronegativity than the hydrogen atoms causing the electrons to spend more time orbiting the oxygen atom. The O-H bonds are polar. Because the shape of the molecule is asymmetrical, the molecule will also be polar.
Some molecules with polar covalent bonds are polar molecules, for example water. But not all molecules with polar covalent bonds are polar, as illustrated in the methane example above. Another example is carbon dioxide. Although CO2 has two polar covalent bonds (between Cδ+ atom and the two Oδ− atoms), the molecule itself is not polar. The reason is that CO2 is a linear molecule, with both terminal atoms the same, and is therefore symmetrical, so there is no difference in charge between the two ends of the molecule.
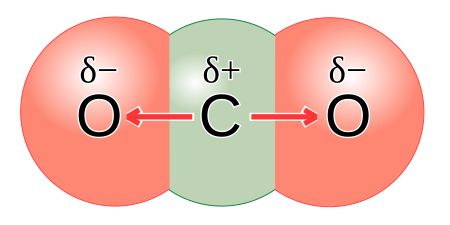
A non-polar molecule can be formed in two ways: firstly, in a molecule with pure covalent bonds where the charge is equally spread across the molecule, for example oxygen, OR secondly, in a molecule with polar covalent bonds and a symmetrical shape where the charge is the same on opposite sides of the molecule, for example carbon dioxide.
Polarity of molecules determines their solubility. In a solution, a solute consisting of polar molecules cannot dissolve in a solvent that has non-polar molecule. For example, consider water and oil. In this solution, water is a polar molecule whereas oil behaves as a non-polar molecule. Fats, petrol, oil, gasoline are non-polar molecules, so they do not dissolve in water. Ionic substances (salts) are classified as polar because of the oppositely charged ions and will therefore dissolve in water. Ethanol has both polar and non-polar characteristics so it will allow both polar and non-polar substances to dissolve in it.
How to determine whether a molecule is polar or non-polar
The following method will help you determine whether a molecule is polar or non-polar.
- Draw a Lewis diagram.
- Use the electronegativity difference to determine if the covalent bonds are polar or pure.
- If the bonds are pure covalent, the molecule will be non-polar.
- If the bonds are polar covalent, use VSEPR to determine the shape of the molecule.
- If the shape is symmetrical (linear with both atoms the same or tetrahedral), the molecule will be non-polar.
- If the shape is asymmetrical (angular or pyramidal), the molecule will be polar.
Example 5.1
Is hydrogen a polar molecule?
Solution
Step 1: Determine the shape of the molecule
The molecule is linear because there is one bonding pair of electrons and no lone pairs.
Step 2: Write down the electronegativities of each atom
Hydrogen: [latex]\scriptsize 2.1[/latex]
Step 3: Determine the electronegativity difference for each bond
There is only one bond and the difference is [latex]\scriptsize \displaystyle 0[/latex].
Step 4: Determine the polarity of each bond
The bond is non-polar (pure). Therefore the molecule is non-polar.
Example 5.2
Is methane a non-polar molecule?
Solution
Step 1: Determine the shape of each molecule
The molecule is tetrahedral because there are four bonding pairs of electrons and no lone pairs.
Step 2: Determine the electronegativity difference for each bond
There are four bonds. Since each bond is between carbon and hydrogen, we only need to calculate one electronegativity difference. This is [latex]\scriptsize 2.5-2.1=0.4[/latex].
Step 3: Determine the polarity of each bond
Each bond is polar because the difference in electronegativity is [latex]\scriptsize 0.4[/latex]. However, the molecule has a tetrahedral shape which is symmetrical, so the molecule is non-polar.
Example 5.3
Is hydrogen cyanide (HCN) a polar or non-polar molecule?
Solution
Step 1: Determine the shape of the molecule
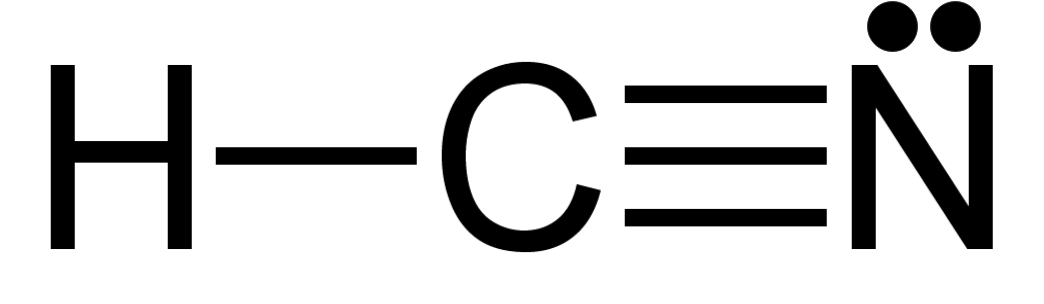
The molecule is linear with different atoms on each end. There are four bonding pairs, three of which form a triple bond and so are counted as one. There is one lone pair on the nitrogen atom.
Step 2: Determine the electronegativity difference and polarity for each bond
There are two bonds. One between hydrogen and carbon and the other between carbon and nitrogen. The electronegativity difference between carbon and hydrogen is [latex]\scriptsize \displaystyle 0.4[/latex] (H side will have a partial positive charge) and the electronegativity difference between carbon and nitrogen is [latex]\scriptsize \displaystyle 0.5[/latex] (N side will have a partial negative charge). Both bonds are polar.
Step 3: Determine the polarity of the molecule
The molecule is not symmetrical because there are two different elements around the central atom, so the molecule is polar.
Exercise 5.1
- Copy the table below into your notebook and complete it:
Geometry Molecule Symmetrical or asymmetrical Polar or non-polar molecule Linear CO2 Linear O2 Bent/angular GeF2 Trigonal pyramidal NH3 - In a molecule of hydrogen chloride:
- What is the electronegativity of hydrogen?
- What is the electronegativity of chlorine?
- Which atom will have a slightly positive charge, and which will have a slightly negative charge in the molecule? Represent this on a sketch of the molecule using symbols for partial charges.
- Is the bond a non-polar or polar covalent bond?
- Is the molecule polar or non-polar?
The full solutions can be found at the end of the unit.
Note
For a further explanation about electronegativity, molecular shape, and polarity you can watch this video, SnapRevise Electronegativity and bond polarity.
Summary
In this unit you have learnt the following:
- Electronegativity is a chemical property that describes the power of an atom to attract bonding pairs of electrons towards itself in a chemical bond.
- Electronegativity can be used to explain the difference between two types of covalent bonds: polar covalent bonds (between non-identical atoms) and non-polar or pure covalent bonds (between identical atoms or atoms with the same electronegativity).
- A polar molecule is one that has one end with a slightly positive charge, and one end with a slightly negative charge. Examples include water, ammonia, and hydrogen chloride.
- A non-polar molecule is one where the charge is equally spread across the molecule or a symmetrical molecule with polar bonds. Examples include carbon dioxide and methane
Unit 5: Assessment
Suggested time to complete: 30 minutes
- Which one of the following molecules is linear?
- Cl2O
- CO2
- SO2
- H2
- Which is the correct description of polarity in F2 and HF molecules?
- Both molecules are polar.
- Only one of the molecules is polar.
- Neither molecule contains a polar bond.
- Both molecules contain a polar bond.
- Which of the following substances is made up of molecules containing polar covalent bonds?
- Water
- Calcium oxide
- Chlorine
- Sodium bromide
- For a covalent bond to be non-polar, the bonding atoms must have the same:
- number of neutrons
- electronegativity
- number of valence electrons
- atomic radius
- The polarity of a bond between two elements can be best determined by:
- The difference in electronegativity between the elements.
- The difference in first ionisation energy between the elements.
- The number of electrons shared in the bond.
- The difference in atomic radius between the elements.
- The ability of an atom to attract bonding pairs of electrons to itself is called:
- Geometry
- Conductivity
- Electronegativity
- Ionisation energy
- What kind of compound is CO2?
- Non-polar molecule with polar bonds
- Polar molecule with polar bonds
- Ionic
- Polar molecule with non-polar bonds
- Phosphine is a compound of phosphorus and hydrogen. It is a highly toxic gas with the formula PH3.
- The shape of a molecule of phosphine is likely to be:
- bent
- pyramidal
- tetrahedral
- linear
- Draw a diagram of PH3, showing its correct shape.
- Is the molecule polar or non-polar? Explain your answer.
- The shape of a molecule of phosphine is likely to be:
- Complete the table below:
Molecule Difference in electronegativity between atoms Non-polar/polar covalent bond Polar/non-polar molecule H2O HBr F2 C2H2
The full solutions can be found at the end of the unit.
Unit 5: Solutions
Exercise 5.1
- .
Geometry Molecule Symmetrical or asymmetrical Polar or non-polar molecule Linear CO2 Symmetrical Non-polar Linear O2 Symmetrical Non-polar Bent/angular GeF2 Not symmetrical Polar Trigonal pyramidal NH3 Not symmetrical Polar - .
- [latex]\scriptsize 2.1[/latex]
- [latex]\scriptsize 3.0[/latex]
- The electronegativity of chlorine is higher than the electronegativity of hydrogen so the electrons will spend more time orbiting the chlorine atom making the chlorine side more negative and the hydrogen side more positive.
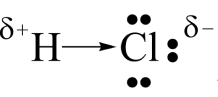
- The bond is polar because the electrons will spend more time around the nucleus of the chlorine atom.
[latex]\scriptsize 3.0-2.1=0.9[/latex] The bond is weakly polar. - The molecule is linear, but it is a polar molecule.
Unit 5: Assessment
- b
- b
- a
- b
- a
- c
- a
- .
- ii
- .
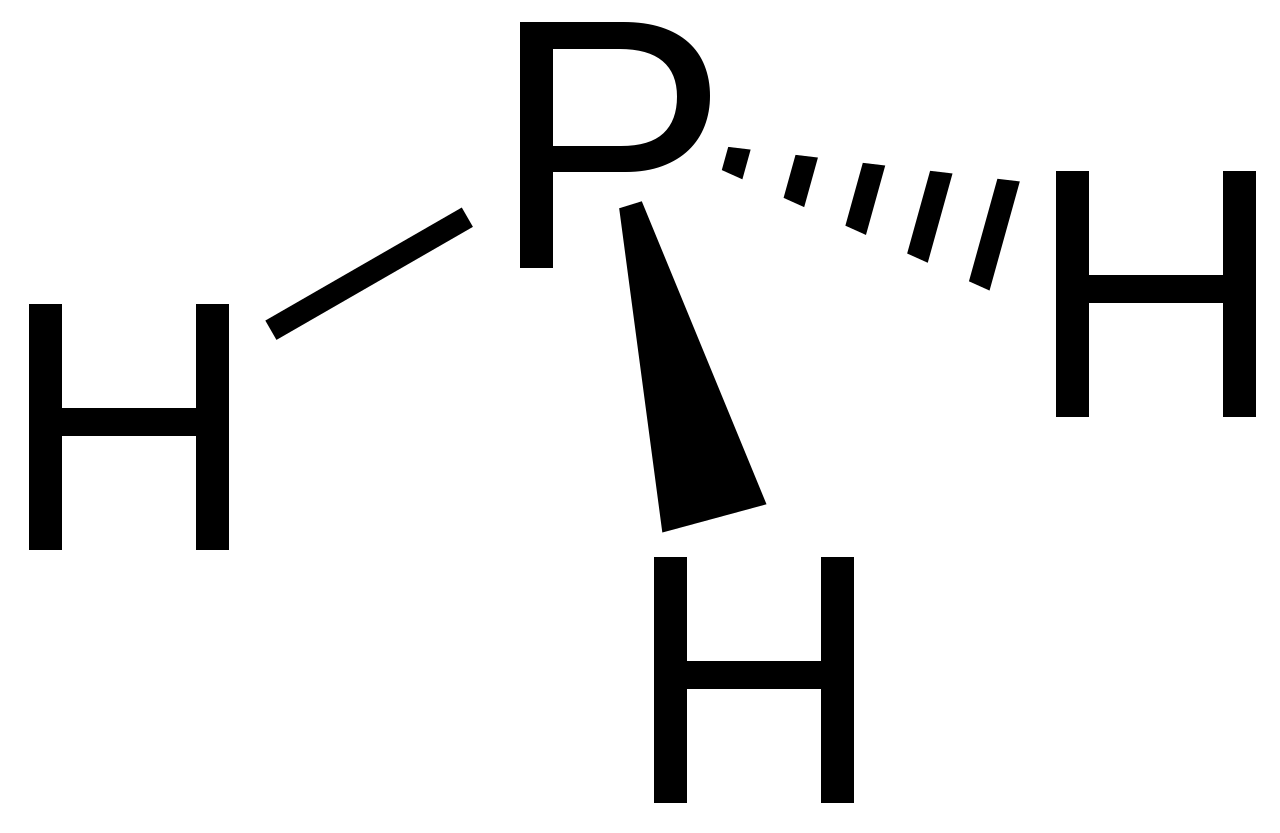
- Electronegativity of P = [latex]\scriptsize 2.1[/latex]
Electronegativity of H = [latex]\scriptsize 2.1[/latex]
[latex]\scriptsize 2.1-2.1=0[/latex]
PH3 is non-polar
- .
Molecule Difference in electronegativity between atoms Non-polar/polar covalent bond Polar/non-polar molecule H2O [latex]\scriptsize 3.5-2.1=1.4[/latex] Polar covalent bond Polar molecule. Water has a bent or angular shape. HBr [latex]\scriptsize 2.8-2.1=0.7[/latex] Polar covalent bond Polar molecule. Hydrogen bromide is linear but not symmetrical. F2 [latex]\scriptsize 4.0-4.0=0[/latex] Pure covalent bond Non-polar molecule C2H2 [latex]\scriptsize 2.5-2.1=0.4[/latex] Polar covalent bond Non-polar molecule. The molecule is tetrahedral and symmetrical.
Media Attributions
- Fig 1 © Rice University is licensed under a CC BY (Attribution) license
- Fig 2 © Riccardo Rovinetti is licensed under a CC BY (Attribution) license
- Fig 3 © openstax is licensed under a CC BY (Attribution) license
- Fig 4 © DHET is licensed under a CC BY (Attribution) license
- Fig 5 © Libretext is licensed under a CC BY-NC (Attribution NonCommercial) license
- Fig 6 © Jü is licensed under a CC0 (Creative Commons Zero) license
- Fig 7 © Sedding Stew is licensed under a CC0 (Creative Commons Zero) license
- Fig 8 © DHET is licensed under a CC BY (Attribution) license
- Fig 9 © Libretext is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Fig 10 © NEUROtiker is licensed under a CC0 (Creative Commons Zero) license
the unequal distribution of charge caused by the difference in electronegativity; this term can be applied to a bond or molecule
the chemical property which describes the power of an atom to attract bonding pairs of electrons towards itself
bonds that occur between atoms that have the same electronegativity
a bond that occurs between atoms that have different electronegative values
a molecule where the charge is equally spread across it or a symmetrical molecule with polar bonds
a molecule that has one end with a slightly positive charge, and one end with a slightly negative charge.
