Chemical Systems and Industry: Identify and critically evaluate the impact of scientific knowledge on the atmosphere and the quality of human, environmental and socio-economic development
Unit 1: The atmosphere
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Understand the chemical properties and uses of carbon dioxide, oxygen, hydrogen, methane, nitrogen, and acetylene.
What you should know:
Before you start this unit, make sure you can:
- Understand the physical properties of a gas in level 2 subject outcome 5.1 unit 1.
- Understand the structure and function of the hydrosphere in level 2 subject outcome 7.1 unit 1.
Introduction
The earth’s atmosphere is made up of many different gases such as oxygen, carbon dioxide, ozone, and hydrogen. The atmosphere is made up of different layers of gas which stretch from the surface all the way out into space and this protective layer of gases shelters all life on Earth, keeping temperatures within a relatively small range and blocking out harmful rays of sunlight. The gases are held near the surface of the planet by Earth’s gravitational attraction. Air pollution caused by industry releasing pollutants such as methane, nitrous oxides and sulphurous oxides cause a variety of problems for humans including asthma, lung disease and different types of cancer.
The importance of the atmosphere
Without the atmosphere, Earth would look a lot more like the moon. Atmospheric gases, especially carbon dioxide (CO2) and oxygen (O2), are extremely important for living organisms.
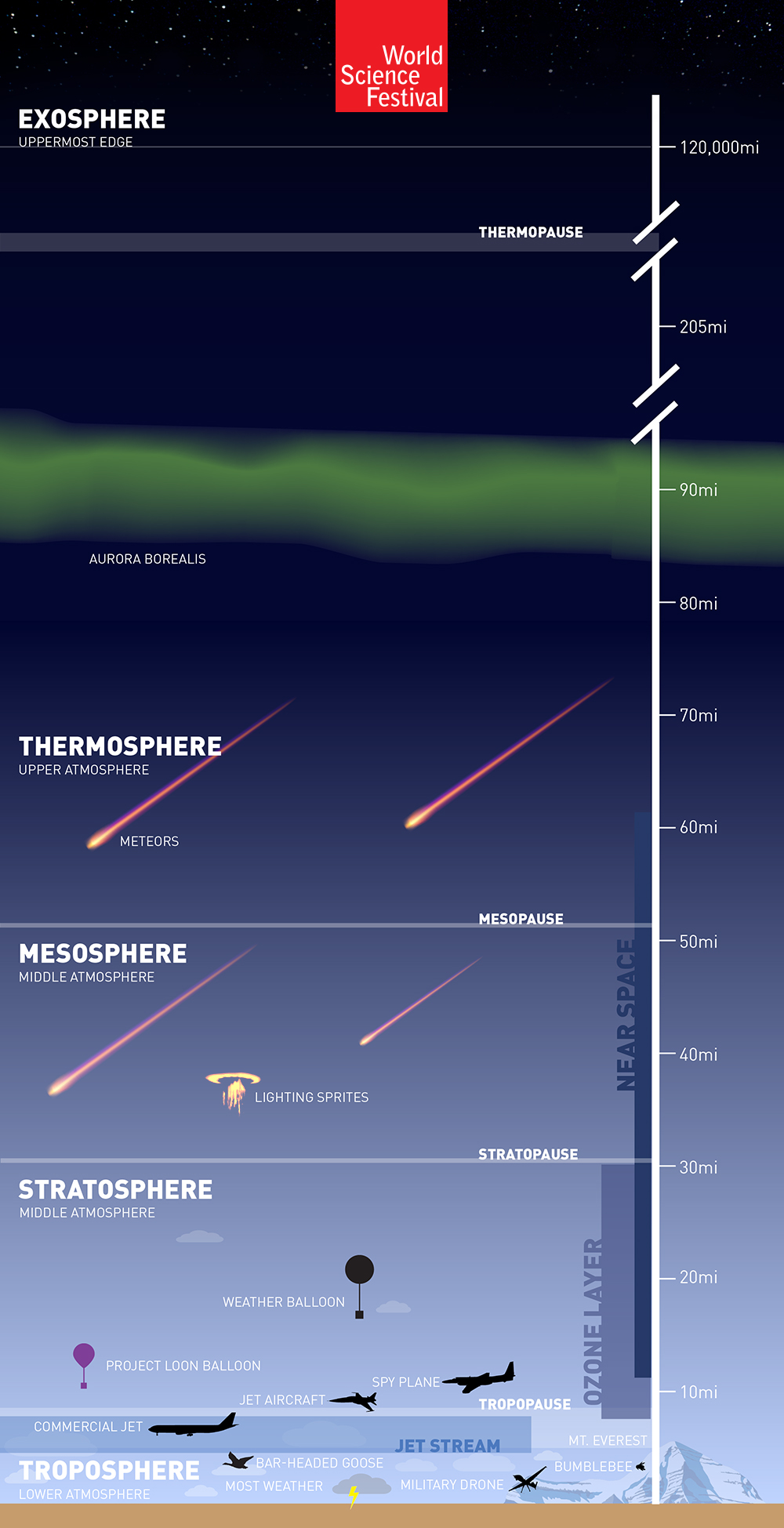
The troposphere is the lowest layer in the atmosphere. It extends upward to about [latex]\scriptsize \displaystyle 10\text{ kms}[/latex] above sea level starting from ground level. The lowest part of the troposphere is called the boundary layer and the topmost layer is called the tropopause. The troposphere contains [latex]\scriptsize \displaystyle 75\%[/latex] of all air in the atmosphere. Most clouds appear in this layer because [latex]\scriptsize \displaystyle 99\%[/latex] of the water vapour in the atmosphere is found here. Temperature and air pressure drop as you go higher in the troposphere.
As part of the hydrologic cycle, water spends a lot of time in the atmosphere, mostly as water vapour. All weather takes place in the atmosphere, virtually all of it in the lower troposphere.
Ozone, which is found in the stratosphere, is a molecule composed of three oxygen atoms, (O3). Ozone in the upper atmosphere absorbs high-energy ultraviolet (UV) radiation coming from the sun. This protects living things on Earth’s surface from the sun’s most harmful rays. Without ozone for protection, only the simplest life forms would be able to live on Earth.
Along with the oceans, the atmosphere keeps Earth’s temperatures within an acceptable range. trap heat in the atmosphere so they help to moderate global temperatures. Without an atmosphere with greenhouse gases, Earth’s temperatures would be frigid at night and scorching during the day. Important greenhouse gases include carbon dioxide, methane, water vapour, and ozone.
Gases in the atmosphere
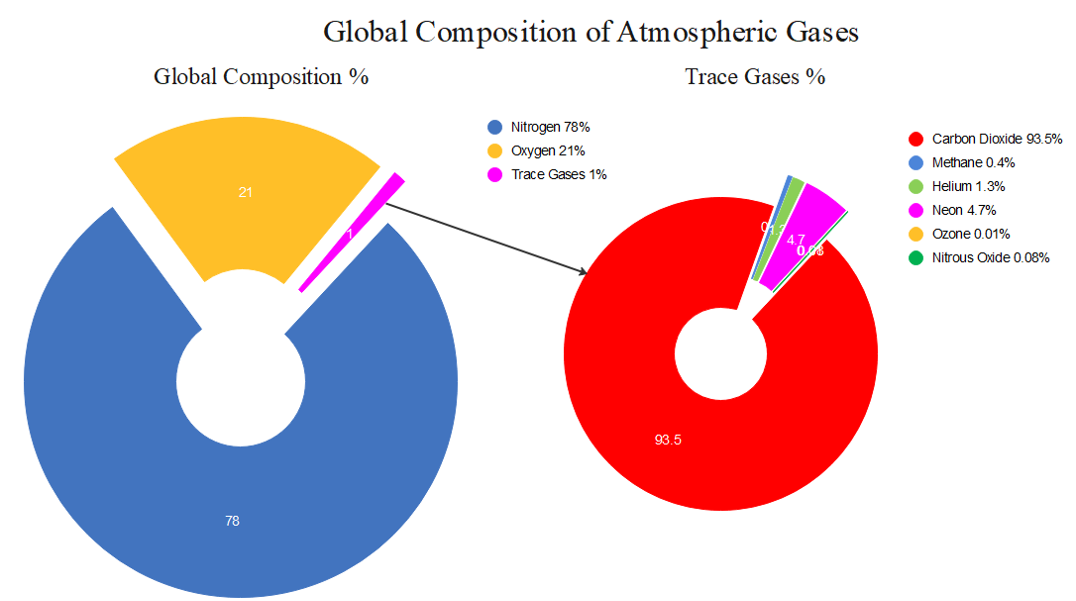
Nitrogen and oxygen together makeup [latex]\scriptsize \displaystyle 99\%[/latex] of the planet’s atmosphere. The rest of the gases are minor components but sometimes are particularly important. Humidity is the amount of water vapour in the air. The amount of water vapour in air varies according to the temperature and density of air. The amount of water vapour ranges from a trace amount to up to [latex]\scriptsize \displaystyle 4\%[/latex] of the mass of air. Hot air can hold more water vapour than cold air, so the amount of water vapour is highest in hot, tropical areas and lowest in cold, polar regions.
Some of what is in the atmosphere is not gas. Particles of dust, soil, metals, salt, smoke, ash, and other solids make up a small percentage of the atmosphere. Particles provide starting points (or nuclei) for water vapour to condense on and form raindrops.
Chemical properties and industrial use of gases
Many naturally occurring gases such as oxygen and nitrogen are used in industry and commercially to manufacture items used in our everyday lives.
Oxygen
Oxygen makes up [latex]\scriptsize \displaystyle 21\%[/latex] of the air and is a colourless odourless gas. Oxygen has two forms, diatomic oxygen(O2) and triatomic ozone (O3).
During , animals, plants and some bacteria take oxygen from the atmosphere and release carbon dioxide, whereas during , green plants use carbon dioxide in the presence of sunlight and give off oxygen. Almost all the free oxygen in the atmosphere is due to photosynthesis. Dissolved oxygen is essential for the respiration of fish and other marine life. Oxygen is necessary for and is added to air to produce oxygen rich air.
When required in large quantities, oxygen is prepared by the fractional distillation of liquid air. The steel industry is the largest consumer of pure oxygen in the manufacture of high carbon steel. Oxygen is needed for the reaction that converts carbon to carbon dioxide gas in steel working, which takes place under high temperatures in a blast furnace. The carbon dioxide produced allows for the reduction of iron oxides into more pure iron compounds.
Oxygen rich air or commercially produced oxygen is used to degrade , which are broken apart by heating them. During combustion, the reaction gives off water and carbon dioxide, but can also produce the hydrocarbons acetylene, propylene, and ethylene.
Oxygen is used in other applications involving metals and requiring high temperatures, such as welding torches. Oxygen is a significant requirement in several industries that use kilns.
Ozone is a powerful , capable of converting sulfur dioxide to sulfur trioxide, sulfides to sulfates, and many organic compounds to oxygenated derivatives such as aldehydes and acids. Commercially, ozone has been used as a chemical reagent, as a disinfectant, in sewage treatment, water purification, and bleaching textiles.
Nitrogen
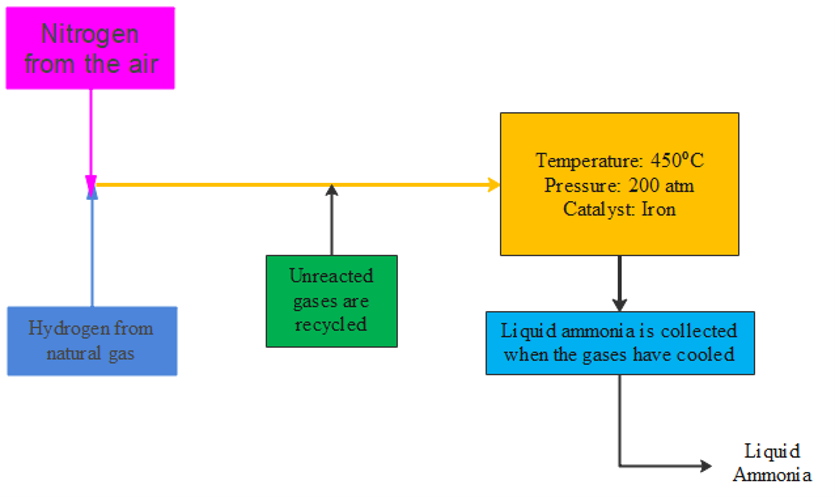
Nitrogen is important to the chemical industry. It is used to make fertilisers, nitric acid, nylon, dyes, and explosives. To make these products, nitrogen must first be reacted with hydrogen to produce ammonia. This is done by the . [latex]\scriptsize \displaystyle 150[/latex] million tonnes of ammonia are produced in this way every year.
Nitrogen gas is also used to provide an unreactive environment to preserve foods, and in the electronics industry during the production of transistors and diodes. Large quantities of nitrogen are used in stainless steel and other steel mill products. Annealing is a heat treatment that makes steel easier to work. Liquid nitrogen is often used as a refrigerant. It is used for storing sperm, ova/eggs and other cells for medical research and reproductive technology. It is also used to rapidly freeze foods, helping them to maintain moisture, colour, flavour, and texture.
Nitrogen is cycled naturally by living organisms through the ‘nitrogen cycle’. It is taken up by green plants and algae as nitrates dissolved in water from the soil and used to build up the bases needed to construct proteins.
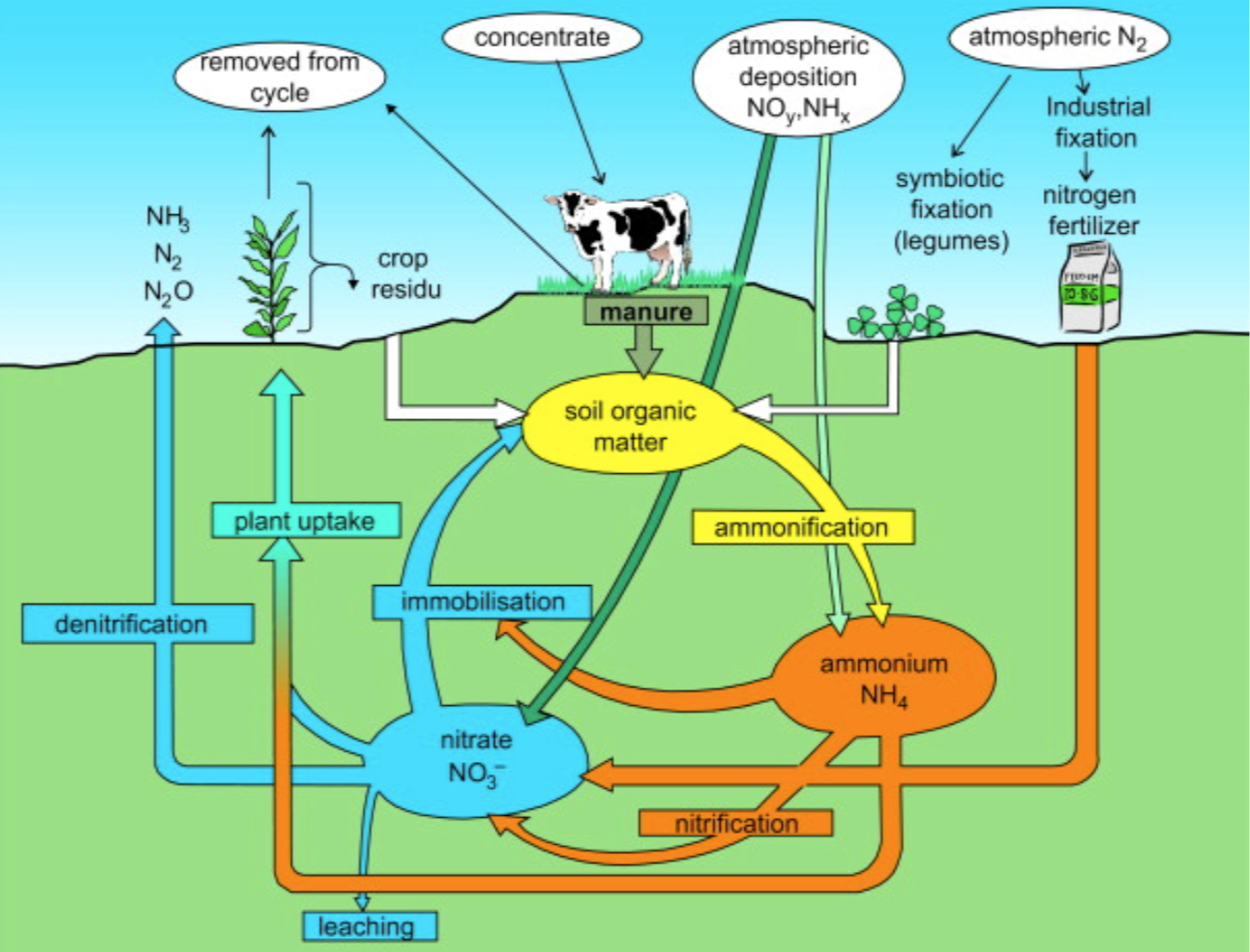
Microbes in the soil convert the nitrogen compounds back to nitrates during decomposition for plants to re-use. Crop yields can be greatly increased by adding chemical fertilisers containing nitrates to the soil. These fertilisers are manufactured from ammonia.
Carbon dioxide
Carbon dioxide is an odourless, colourless gas that is stable at room temperature. Living creatures produce carbon dioxide as a waste product of respiration, which is then utilised by plants to form food by photosynthesis.
Carbon dioxide gas is used in industries to produce chemicals. Carbon dioxide gas is involved in the production of refrigeration systems, welding systems, water treatment processes (to stabilise the pH of water) and carbonated beverages. Carbon dioxide is soluble in water. When it dissolves it forms carbonic acid. Carbonic acid is what gives fizzy drinks their bubbles. At high pressures, more carbon dioxide dissolves in water, and because this reaction is reversible, when the pressure decreases carbon dioxide is released again. This explains why bubbles appear in a bottle of sparkling water when you unscrew the lid.
Carbon dioxide is also used in the metals industry to enhance the hardness of casting moulds and as a soldering agent. Carbon dioxide is found in various fire extinguishers and prevents oxygen from further fuelling a fire. Carbon dioxide is denser than air. It sinks, which means that it can smother a fire – starving the fire of oxygen and putting it out. This is why many fire extinguishers contain carbon dioxide gas to manage electrical fires and those caused by solvents, fuels, and oils.
Carbon dioxide gas is used to make urea, methanol, inorganic and organic carbonates, polyurethanes, and sodium salicylate. Carbon dioxide is combined with epoxides to create plastics and polymers. It is used for water treatment; to keep food cool (as dry ice); and to cool, pressurise and purge equipment.
Carbon dioxide gas is used in the electronics industry for circuit board assembly, to clean surfaces and in the manufacture of semiconductor devices.
Carbon dioxide gas is used in enhanced oil recovery. Carbon dioxide is injected under high pressure into an oil reservoir, which pushes the oil through pipes and up to the surface of the ground. Carbon dioxide gas injection aids oil recovery and reduces the viscosity of recovered oil.
Carbon dioxide is also a potent greenhouse gas and causes the increased acidity of oceans.
Hydrogen
Hydrogen gas (H2) is a colourless, odourless gas. It has the lowest density of all gases. It is highly flammable and will burn in air at a very wide range of concentrations. Hydrogen has many chemical and industrial uses.
A common use of hydrogen gas is in the gas welding process. It is used in this type of welding to generate a high temperature of [latex]\scriptsize \displaystyle 4000^\circ \text{C}[/latex]. This high temperature leads to the melting of metals and thereby joining broken surfaces. Besides generating heat, hydrogen also acts as a shielding gas. Since metals at high temperatures are very reactive, hydrogen prevents them from reacting with other elements such as nitrogen and carbon during the process. This process is also called atomic hydrogen welding.
Hydrogen is commonly used as fuel for automobiles. Hydrogen fuel is a zero-emission fuel that burns on reaction with oxygen. It is an exciting concept which aims to power vehicles by use of hydrogen instead of petroleum fuels. This technology is being developed for use on a large scale as a substitute for petroleum oil-based engines because burning hydrogen as a fuel does not cause air pollution.
Hydrogen is also used in petroleum refining. Hydrogen gas is widely used in the petroleum industry to remove sulfur. It is also used to break long-chain hydrocarbons into shorter chains.
Hydrogen gas is used in ultraviolet lamps, such as deuterium arc lamps, in the form of . The lamps have a tungsten filament, which generates heat. The heat in the bulb excites the deuterium atoms (2H), which produce ultraviolet light.
One of the first uses of hydrogen gas was in flying hot balloons in the air. Due to its lightweight and low cost, hydrogen gas was used in the air balloons for flight. But due to its explosiveness, it has been replaced with helium, a more stable gas.
As rocket fuel for space programs, hydrogen is a powerful propellant for rockets. It has the lowest molecular weight and is said to burn with extreme intensity.
Hydrogen is used in mass destruction bombs. The explosion relies on the principle of nuclear fusion of hydrogen atoms isotopes. Hydrogen bombs are [latex]\scriptsize \displaystyle 1\text{ }000[/latex] times more powerful than an atomic bomb.

Hydrogen bonds with oxygen to form hydrogen peroxide (H2O2). Hydrogen peroxide is used as a sterilising agent in clinics and hospitals because it kills bacteria.
Methane
Methane (CH4) is a colourless, odourless gas. Methane is the primary component of natural gas, which is a common fuel source. It is widely used to power homes, turbines, and vehicles. Methane can also be liquefied for ease of storage or transport. When combined with liquid oxygen, refined liquid methane can serve as a source of fuel for rockets.
Natural gas is also used to produce hydrogen gas on an industrial scale. Hydrogen is then used for manufacturing ammonia, which is used for fertilisers and explosives. The carbon black generated upon incomplete combustion of methane is a reinforcing agent for rubber in tires.
Some of the most important chemical reactions involving methane are combustion because of the amount of heat energy it releases when it is burnt.
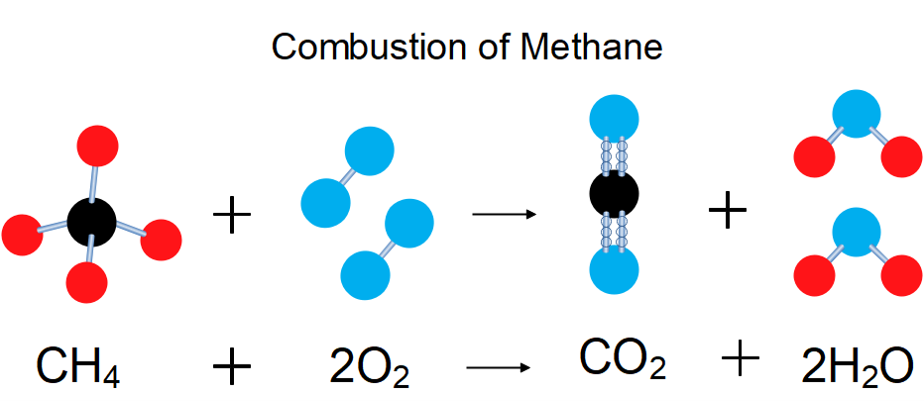
Methane is the cleanest burning fossil fuel and constitutes most of natural gas. Although methane is relatively stable, it can be explosive when its content is between [latex]\scriptsize \displaystyle 5\%[/latex] and [latex]\scriptsize \displaystyle 14\%[/latex] in air, and it has been the cause of many mine disasters. Methane is also a potent greenhouse gas.
Acetylene
Acetylene is a chemical compound composed of two carbon and two hydrogen atoms (C2H2) and is slightly soluble in water. Acetylene is best known as the simplest of all alkynes. It is a colourless gas that has an odour similar to garlic. In its normal state, it can undergo a phase change from gas to liquid; but when heated or exposed to, it may explode.
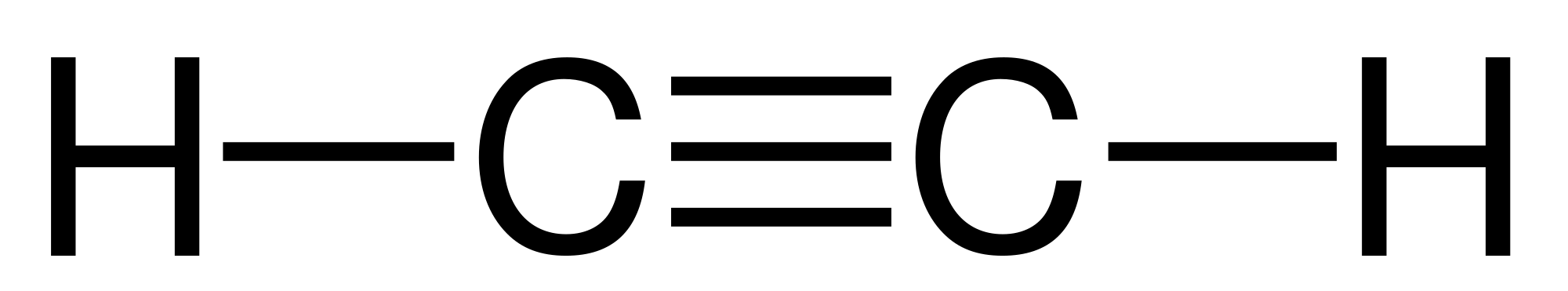
Acetylene is manufactured by subjecting methane to partial combustion in a process called thermal cracking. Methane is heated to the point where the atomic bonds break, or crack. After the hydrocarbon atoms break apart, they can be made to rebind to form different materials.
Acetylene can also be prepared from the of calcium carbide (CaC2). This reaction produces a considerable amount of heat, which must be removed to prevent the acetylene gas from exploding. This process is called wet processing because an excess amount of water is used to absorb the heat of the reaction.
[latex]\scriptsize \displaystyle {{\text{H}}_{2}}\text{O + Ca}{{\text{C}}_{2}}\text{ }\to \text{ }{{\text{C}}_{2}}[/latex]
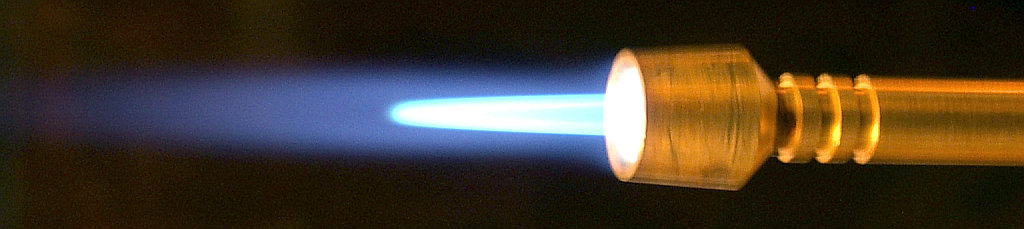
Since acetylene burns with an extremely hot flame, the most common use of acetylene is in oxyacetylene gas welding and oxyacetylene gas cutting. When acetylene is subjected to combustion with oxygen, the flame created is known to have a temperature of roughly [latex]\scriptsize \displaystyle 3600\text{ K/ 3 327}{{\text{ }}^{0}}\text{C}[/latex].
Acetylene is also used in the production of several polyethene plastics.
Because acetylene is highly explosive, it must be stored and handled with great care. When it is transported through pipelines, the pressure is kept very low, and the length of the pipeline is short. In most chemical production operations, the acetylene is transported only as far as an adjacent plant. When acetylene must be transported for use in oxyacetylene welding and metal cutting operations, it is pressurised and stored in special storage cylinders. The cylinders are filled with an absorbent material, such as crushed silica rock, and a small amount of acetone. The acetylene is pumped into the cylinders at high pressure, and it dissolves in the acetone. Once dissolved, it loses its explosive capability, making it safe to transport.
Summary
In this unit you have learnt the following:
- The troposphere is the lowest level of the atmosphere.
- The troposphere contains most of the air found in the atmosphere and where most weather and the hydrologic cycle take place.
- The air is made up of the gases nitrogen, oxygen, carbon dioxide and other trace gases which are useful for industrial processes.
- Nitrogen is used to make explosives, ammonia (the Haber Process) and to preserve food.
- Carbon dioxide is used in feedstock, refrigerants, carbonated soft drinks and other useful chemicals.
- Methane is found in natural gas and is used domestically and industrially as a fuel and in the production of hydrogen.
- Hydrogen has many industrial uses, including welding and as a fuel for vehicles.
- Oxygen is needed for combustion and for respiration to occur and is produced during photosynthesis.
- Acetylene is a hydrocarbon manufactured from methane and is used in welding because it burns with an extremely hot flame.
Unit 1: Assessment
Suggested time to complete: 15 minutes
- State the two main gases found in the air.
- List an industrial use for:
- Methane
- Nitrogen
- Acetylene
- Briefly explain the Haber Process. You may use a flow diagram as your explanation.
- Which gas is used to produce hydrogen on an industrial scale? State the uses of the hydrogen produced.
- Identify the advantage of using an acetylene torch to weld versus a hydrogen torch.
The full solutions can be found at the end of the unit.
Unit 1: Solutions
Unit 1: Assessment
- Nitrogen and oxygen.
- .
- Methane – source of fuel
- Nitrogen – to manufacture ammonia (or any of the following: explosives, fertiliser, nitric acid, unreactive atmosphere, annealing)
- Acetylene – fuel for welding torches
- The Haber process

Figure 4: A simplified flow diagram showing how the Haber process is used to manufacture ammonia - Methane is used to produce hydrogen. Hydrogen is used in explosives, as a fuel, for welding, in petroleum refining, in UV lamps and balloons.
- Acetylene torches burn with a much hotter flame than a hydrogen flame.
Media Attributions
- Fig 3 © NASA is licensed under a Public Domain license
- Fig 1 © WSF is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
- Fig 5 © DHET is licensed under a CC BY (Attribution) license
- Fig 4 © Chem Libre Text is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 6 © The Official CTBTO Photostream is licensed under a Public Domain license
- Fig 7 © DHET is licensed under a CC BY (Attribution) license
- Fig 9 is licensed under a Public Domain license
- Fig 8 © Rnbc is licensed under a CC BY-SA (Attribution ShareAlike) license
a gas that absorbs and emits radiant energy causing the greenhouse effect in the atmosphere
process where organisms use oxygen to oxidise sugars to generate energy
a process used by plants and other organisms to convert light energy into chemical energy
burning in oxygen
organic compounds consisting entirely of hydrogen and carbon
a reactant that removes electrons from other reactants during a redox reaction; the oxidising agent typically takes these electrons for itself, thus gaining electrons and being reduced
a nitrogen fixation process used to make ammonia
a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable
one of two stable isotopes of hydrogen
any chemical reaction in which water is used to break one or more chemical bonds
